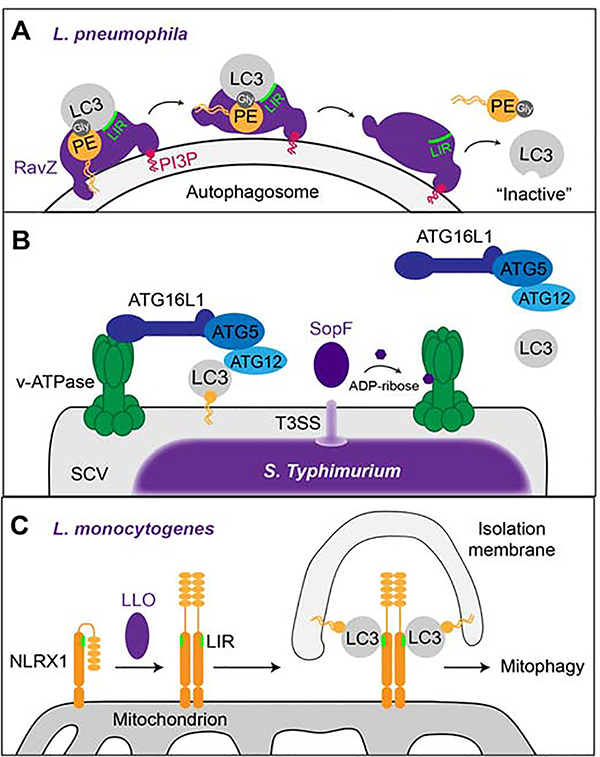
Figure 3: Manipulation of autophagic pathways by intracellular pathogens.
(A) The L.p. effector RavZ binds to PI3P on membranes (e.g. autophagosome) and binds to PE-anchored LC3 via its LIR motif. The N-terminal α-helix of RavZ then moves the PE moiety towards its catalytic site where the bond between LC3 and PE is irreversibly cleaved at the terminal glycine residue, thereby releasing LC3 proteins that cannot be re-lipidated. (B) Upon bacterial infection, the v-ATPase on the bacterial vacuole recruits ATG16L1 to induce xenophagy via ATG5- ATG12-mediated lipidation and membrane anchoring of LC3. The T3SS-secreted S. Typhimurium effector SopF ADP-ribosylates the v-ATPase on the SCV which prevents recruitment of the autophagic machinery and, hence, xenophagy. (C) The L. monocytogenes toxin LLO induces the oligomerization of NLRX1 on host cell mitochondria which leads to a conformational shift that exposes the LIR motif on NLRX1. This enables the binding of LC3 to NLRX1 and mitophagy.