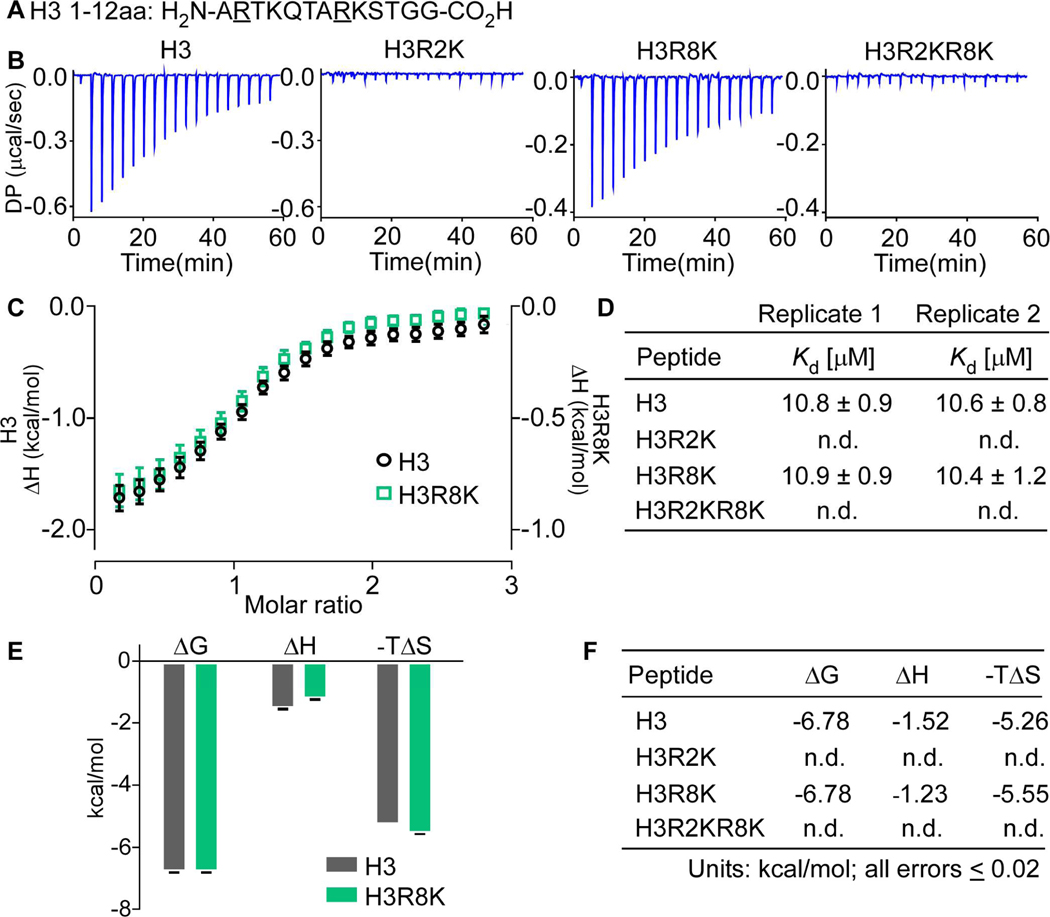
Figure 2.
Replicate isothermal calorimetry assays showing WDR5 interacts with specifically with H3R2. a. Histone H3 1–12aa peptide sequence with R to K mutant positions underlined b. Raw power differentials (DP) of peptide titrations c. Integrated heats of binding; error bars represent the standard error between two replicate titrations d. Dissociation constants for replicate titrations determined by fitting heat integrations to a one-site binding model e. Histogram of thermodynamic parameters for interacting peptides (ΔG, Gibbs free energy change; ΔH, enthalpy change; T, temperature in Kelvin; ΔS, entropy change) f. Tabulated thermodynamic parameters for interacting peptides (n.d., not detected). Peptides bound WDR5 with stoichiometries (N-values) between 0.9 and 1.1.