Abstract
Background:
Androgen deprivation therapy (ADT) is extensively used in prostate cancer. Yet the risk of impaired cognition or Alzheimer disease (AD) in men with prostate cancer receiving ADT is uncertain. Some studies of prostate cancer and ADT suggest that the risk of AD is not increased. But other studies have found an increased risk of AD and cognitive impairment.
Objectives:
As the uncertainty about ADT and dementia might relate to the genetics of prostate cancer and AD, the authors used the Cancer Genome Atlas (TCGA) to examine the relationship in men with prostate cancer between genes implicated in AD and genes implicated in prostate cancer.
Methods:
The authors examined the genomics of 492 prostate cancer cases in the Genomic Data Commons (GDC) TCGA Prostate Cancer (PRAD) data set. To access and analyze the data, 2 web-based interfaces were used: (1) the UCSC Xena browser, a web-based visual integration and exploration tool for TCGA data, including clinical and phenotypic annotations; and (2) cBioportal, a web-based interface that enables integrative analysis of complex cancer genomics and clinical profiles.
Results:
Co-occurrence analysis indicates that alterations in the prostate cancer gene Speckle-type POZ protein (SPOP) significantly co-occur with alterations in the AD gene BIN1 (P < 0.001). The presence of somatic mutations (deleterious and missense/in frame) in SPOP deranges BIN1 gene expression. SPOP/BIN1 RNA gene expression in 492 prostate cancer specimens is significantly correlated (P < 0.001). Increased expression of SPOP in 492 prostate cancers is associated with reduced survival (P = 0.00275). Men receiving pharmacologic therapy had a tumor with a significantly higher Gleason score (P = 0.023). Gleason score and BIN1 RNA gene expression, unit log2 (fragments per kilobase of transcript per million mapped reads upper quartile [FPKM-UQ]+1), in 499 prostate cancer specimens were significantly inversely correlated (P < 0.001).
Conclusions:
BIN1 forms part of a network that interacts with the MYC oncogene, activated at the earliest phases of prostate cancer and in its position on chr8q24 linked to disease aggressiveness. Dynamic regulation of the BIN1-Tau interaction is involved in AD. BIN1 loss in AD allows phosphorylated tau to be mis-sorted to synapses, which likely alters the integrity of the postsynapse, alongside reducing the functionally important release of physiological forms of tau. Alzheimer symptoms are usually preceded by a preclinical phase that may be 16 years long. The authors suggest that the ADT dosage reflects the severity of a process that is already underway. The severity is determined by the genetics of the tumor itself, at least in part by BIN1. ADT is not causing new cases of AD. The oncologist treats higher-grade prostate cancer with more ADT, which serves as a surrogate marker for disease severity. Our analysis of TCGA data does not support the idea that ADT causes AD or dementia.
Keywords: genomics, dementia, The Cancer Genome Atlas, prostate, carcinoma
Androgen deprivation therapy (ADT) is extensively used in prostate cancer. Yet the risk of impaired cognition or Alzheimer disease (AD) in men with prostate cancer receiving ADT is uncertain.1 Some studies of prostate cancer and ADT suggest that the risk of AD is not increased.2–6 But other studies have found an increased risk of AD and cognitive impairment.7–9
The androgen receptor (AR) directly mediates neuroprotection.10 But testosterone apparently does not affect those parts of the brain that demonstrate sex differences in performance; and no one knows whether testosterone is necessary to maintain intellect throughout life.11
Testosterone is related to cognition, and sex hormones affect brain development. Androgens modify neural activity needed for learning and memory and are neuroprotective during aging. Androgens protect against AD in mouse models12 and, hypothetically, humans.13
The uncertainty about ADT and dementia may relate to the genetics of prostate cancer and AD. In the current analysis, we used the Cancer Genome Atlas (TCGA) to examine the relationship in men with prostate cancer between genes implicated in AD and genes implicated in prostate cancer.
METHODS
We examined the genomics of prostate cancer in the Genomic Data Commons (GDC) TCGA Prostate Cancer (PRAD) data set and the MSKCC/DFCI data set.14 TCGA contains an analysis of over 11,000 tumors from 33 of the most prevalent forms of cancer.15 To access and analyze the data we used:
UCSC Xena browser, a web-based visual integration and exploration tool for TCGA data, including clinical and phenotypic annotations.16 Gene expression is quantitated as fragments per kilobase of transcript per million mapped reads upper quartile (FPKM-UQ), which is an RNA-Seq-based expression normalization method.17
cBioportal, a web-based interface that enables integrative analysis of complex cancer genomics and clinical profiles.18
PCViz, an open-source web-based network visualization tool that helps users obtain details about genes and their interactions extracted from multiple pathway data resources.
Simple statistics were calculated to identify patterns of mutual exclusivity or co-occurrence. For a pair of query genes, an odds ratio is calculated (equation 1) that indicates the likelihood that the events in the 2 genes are mutually exclusive or co-occurrent across the selected cases
| (1) |
where A = number of cases altered in both genes; B = number of cases altered in gene A but not gene B; C = number of cases altered in gene B but not gene A; and D = number of cases altered in neither gene. Each pair was then assigned to one of 3 categories indicative of a tendency toward mutual exclusivity, of a tendency toward co-occurrence, or of no association. To determine whether the identified relationship is significant for a gene pair, the Fisher exact test was performed.18 A q-value is derived from the Benjamini Hochberg false discovery rate correction procedure for multiple comparisons.
Survival data of patients with prostate cancer with primary solid tumors were used to generate Kaplan-Meier curves of overall survival.19 Survival time was defined as the period from the date of diagnosis to the date of death. If unavailable, then the date of the last follow-up was used for KM right censoring. Differences between Kaplan-Meier survival curves were calculated by the log-rank (Mantel-Cox) test.
RESULTS
Patients with prostate cancer with primary tumors were aged 61 ± 6.8 years (mean ± SD). Thirty percent were white, 1.4% African American, and 0.4% Asian; of the remainder, the race was not recorded. Ninety-seven percent were prostate adenocarcinoma acinar type.
Co-occurrence analysis (Table 1) indicates that alterations in the prostate cancer gene Speckle-type POZ protein (SPOP) significantly co-occur with alterations in the AD gene Bridging integrator 1 (BIN1) (P < 0.001, q < 0.001). Alterations in the prostate cancer gene Spectrin Alpha, Erythrocytic 1 (SPTA1) significantly co-occur with alterations in the AD gene CD2-associated protein (CD2AP) (P < 0.001, q = 0.004).
TABLE 1.
Significantly Co-occurring Alterations in Patients With Prostate Cancer of SPOP, SPTA1, AR, APOE, BIN1, and CD2AP Genes
| A | B | Neither | A Not B | B Not A | Both | Log2 Odds Ratio | P | q | Tendency |
|---|---|---|---|---|---|---|---|---|---|
| SPOP | BIN1 | 425 | 46 | 7 | 11 | > 3 | < 0.001 | < 0.001 | Co-occurrence |
| APOE | CD2AP | 483 | 2 | 1 | 3 | > 3 | < 0.001 | < 0.001 | Co-occurrence |
| SPTA1 | CD2AP | 459 | 26 | 1 | 3 | > 3 | < 0.001 | 0.004 | Co-occurrence |
| SPTA1 | APOE | 457 | 27 | 3 | 2 | > 3 | 0.03 | 0.114 | Co-occurrence |
| CD2AP | AR | 479 | 3 | 6 | 1 | > 3 | 0.056 | 0.169 | Co-occurrence |
| APOE | AR | 478 | 4 | 6 | 1 | > 3 | 0.07 | 0.175 | Co-occurrence |
| BIN1 | CD2AP | 468 | 17 | 3 | 1 | > 3 | 0.14 | 0.299 | Co-occurrence |
| BIN1 | AR | 465 | 17 | 6 | 1 | 2.189 | 0.232 | 0.435 | Co-occurrence |
| SPOP | SPTA1 | 405 | 55 | 27 | 2 | −0.874 | 0.319 | 0.532 | Mutual exclusivity |
| SPOP | AR | 425 | 57 | 7 | 0 | < −3 | 0.418 | 0.626 | Mutual exclusivity |
| SPOP | APOE | 427 | 57 | 5 | 0 | < −3 | 0.537 | 0.732 | Mutual exclusivity |
| SPOP | CD2AP | 428 | 57 | 4 | 0 | < −3 | 0.608 | 0.75 | Mutual exclusivity |
| SPTA1 | AR | 453 | 29 | 7 | 0 | < −3 | 0.65 | 0.75 | Mutual exclusivity |
| SPTA1 | BIN1 | 443 | 28 | 17 | 1 | −0.104 | 0.71 | 0.761 | Mutual exclusivity |
| APOE | BIN1 | 466 | 5 | 18 | 0 | < −3 | 0.828 | 0.828 | Mutual exclusivity |
q-value is derived from the Benjamini Hochberg false discovery rate correction procedure for multiple comparisons.
Co-occurring alterations in the gene pairs SPOP/BIN1 and SPTA1/CD2AP are illustrated in the Oncoprint diagram (Fig. 1), along with APOE and AR (androgen receptor gene) alterations. A heatmap showing gene expression is presented in Figure 2.
FIGURE 1.
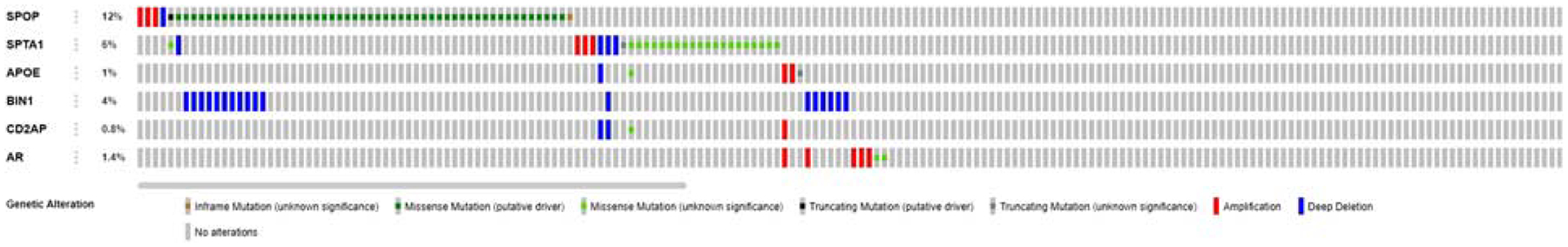
Oncoprint diagram: SPOP, SPTA1, AR (prostate cancer related), APOE, BIN1, and CD2AP (Alzheimer’s disease related) in 489 primary prostate cancer samples. Alterations are present in 12% of SPOP, 4% of BIN1, 6% of SPTA1, 0.8% of CD2AP, 1% of APOE, and 1.4% of AR. Significantly co-occurrent alterations are present in the gene pairs SPOP/BIN1 and SPTA1/CD2AP (cBioportal).
FIGURE 2.
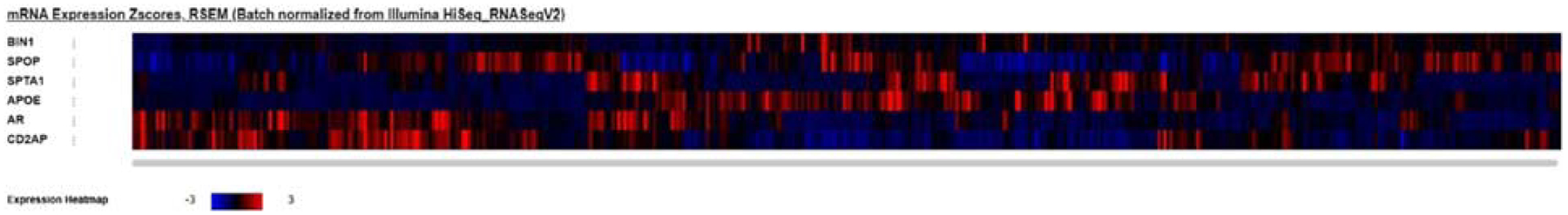
Expression heatmap of SPOP, SPTA1, AR (prostate cancer related), APOE, BIN1, and CD2AP (Alzheimer disease related) in 489 primary prostate cancer samples.
SPOP somatic mutations and BIN1 gene expression in 492 primary prostate cancer samples are shown in Figure 3. Note that the presence of somatic mutations (deleterious and missense/in frame) in SPOP deranges BIN1 gene expression.
FIGURE 3.
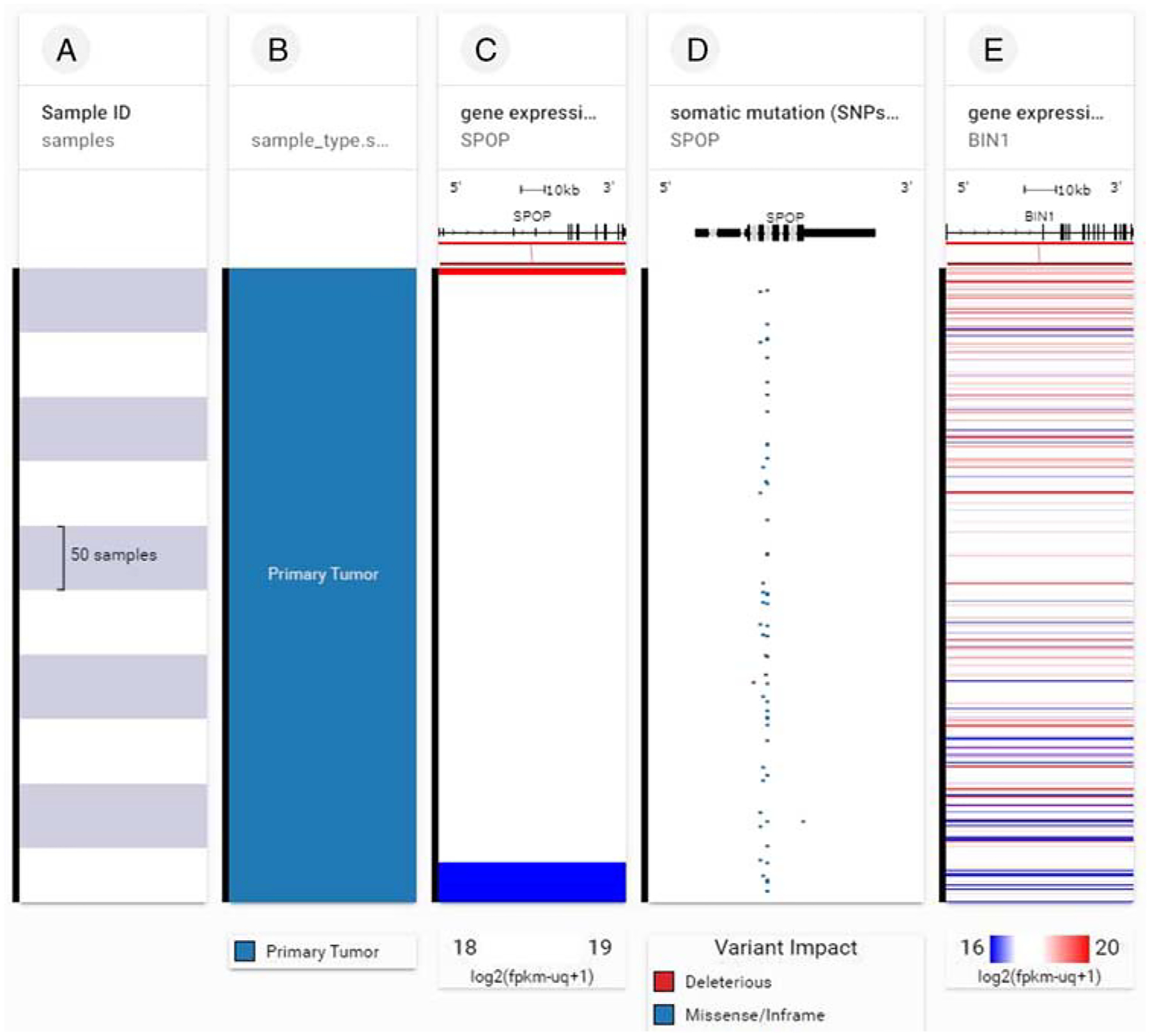
SPOP gene expression, SPOP somatic mutations, and BIN1 gene expression in 492 primary prostate cancer samples. Column A indicates number of samples, column B indicates that all samples were primary tumor. Note that the presence of somatic mutations (deleterious and missense/in frame) in SPOP (column D) deranges BIN1 gene expression (multicolored horizontal lines, column E). Each row contains data from a single sample. Row order is determined by sorting the rows by their column values. The column C value is used to sort the rows. (xenabrowser.net).
SPOP/BIN1 gene RNA expression in 492 prostate cancer specimens is significantly correlated (P < 0.001, Fig. 4).
FIGURE 4.
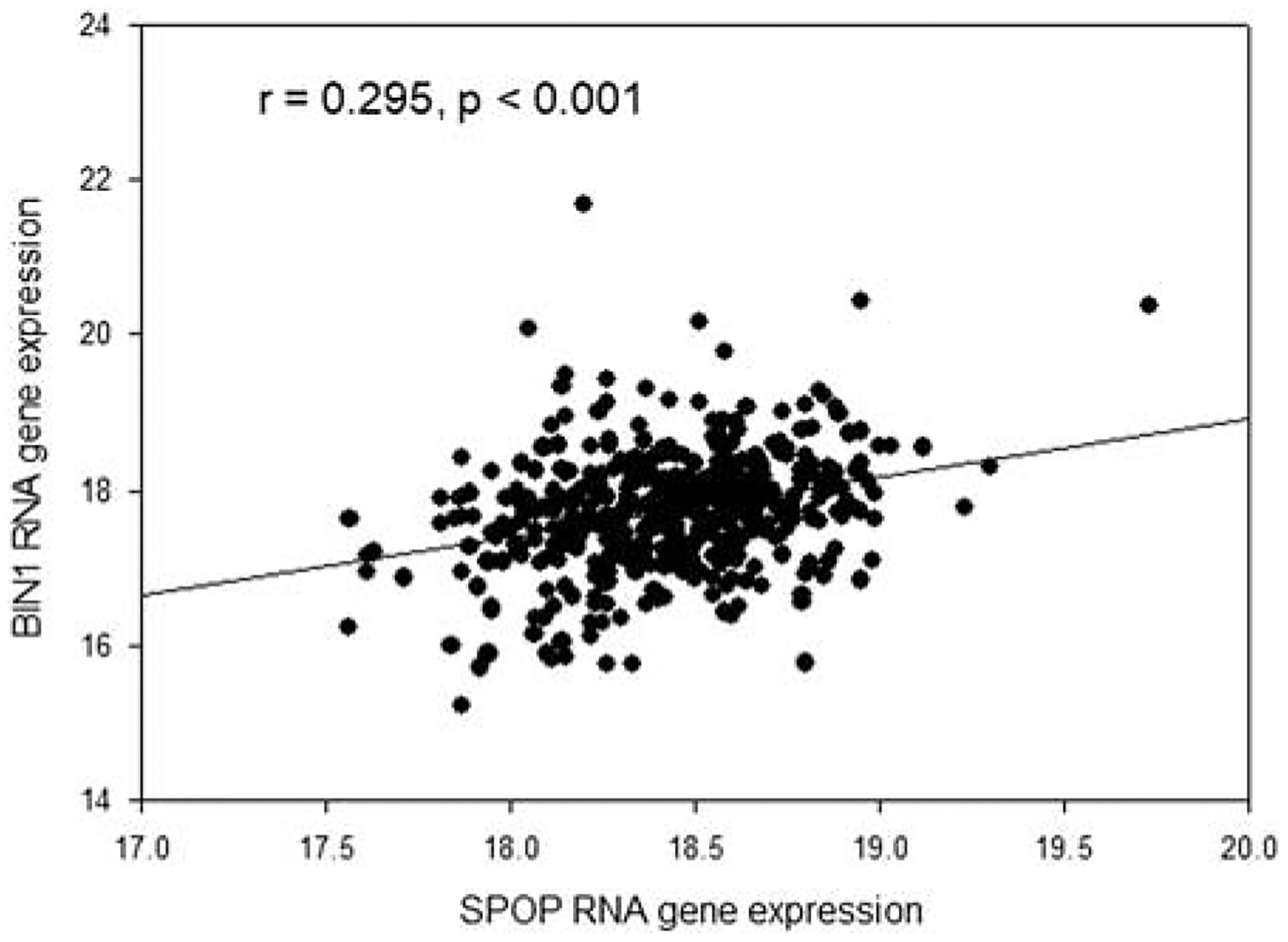
SPOP/BIN1 RNA gene expression in 492 prostate cancer specimens, unit log2 (FPKM-UQ+1).
Figure 5 shows Gleason score/BIN1 RNA gene expression, unit log2 (FPKM-UQ+1), in 499 prostate cancer specimens. The correlation is significant.
FIGURE 5.
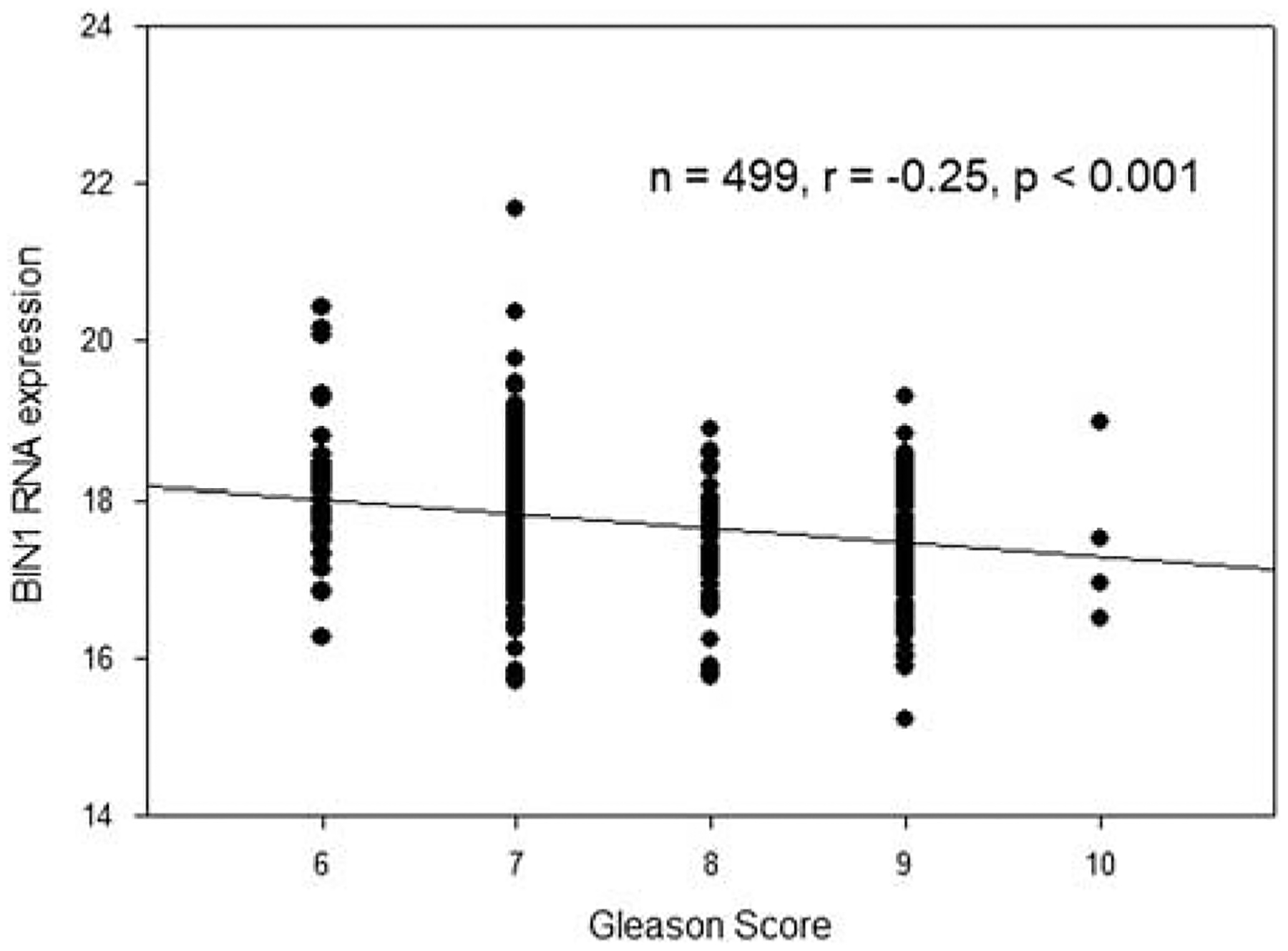
Gleason score/BIN1 RNA gene expression, unit log2 (FPKM-UQ+1), in 499 prostate cancer specimens. FPKM-UQ indicates fragments per kilobase of transcript per million mapped reads upper quartile.
Increased expression of SPOP in 492 prostate cancers is associated with reduced survival (P = 0.00275, Fig. 6).
FIGURE 6.
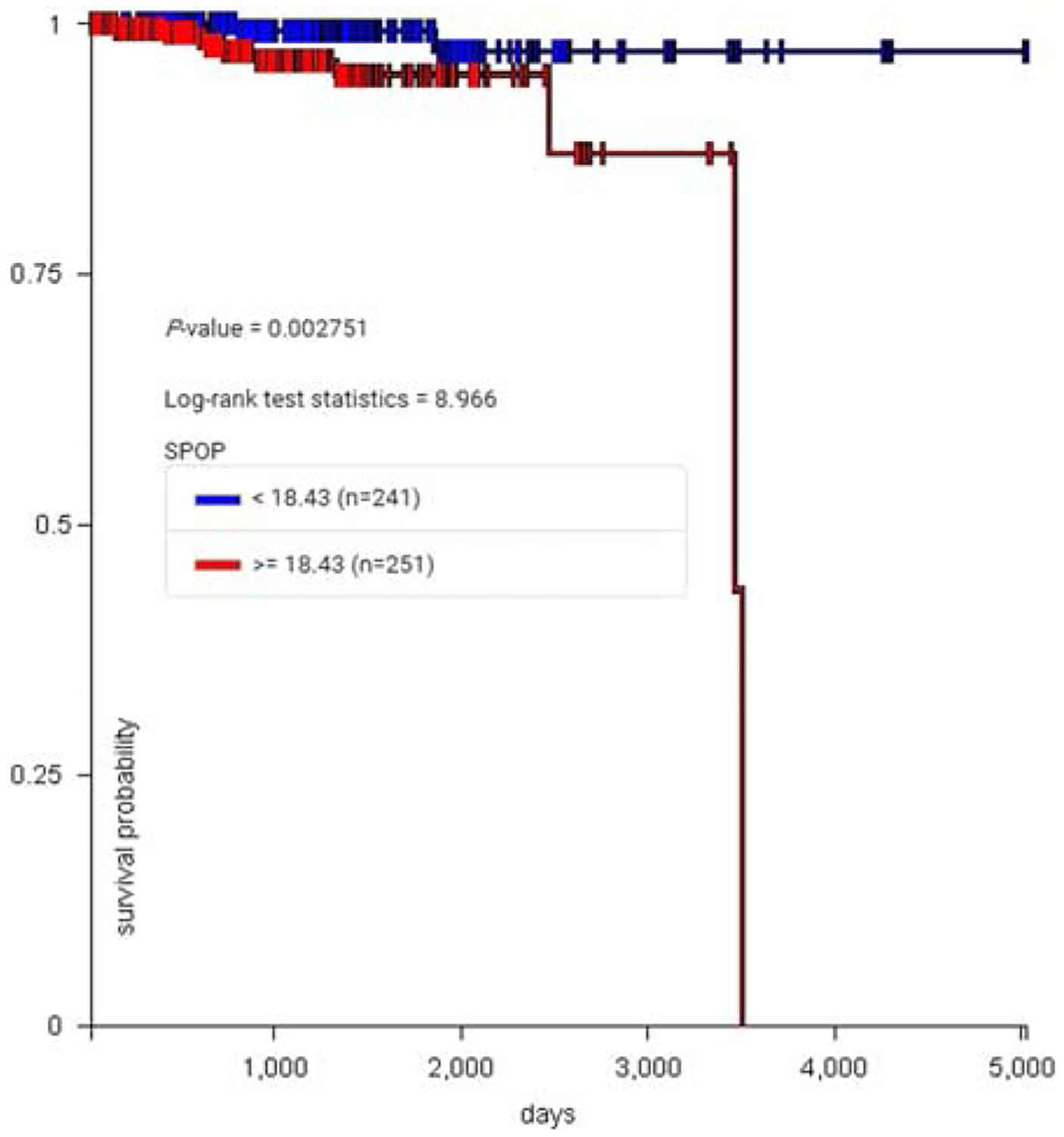
Increased expression of SPOP in 492 prostate cancers (red) is associated with reduced survival (P = 0.00275).
Mean Gleason score of patients with prostate cancer versus pharmacologic therapy is shown in Figure 7. Those receiving pharmacologic therapy had a tumor with a significantly higher Gleason score (P = 0.023).
FIGURE 7.
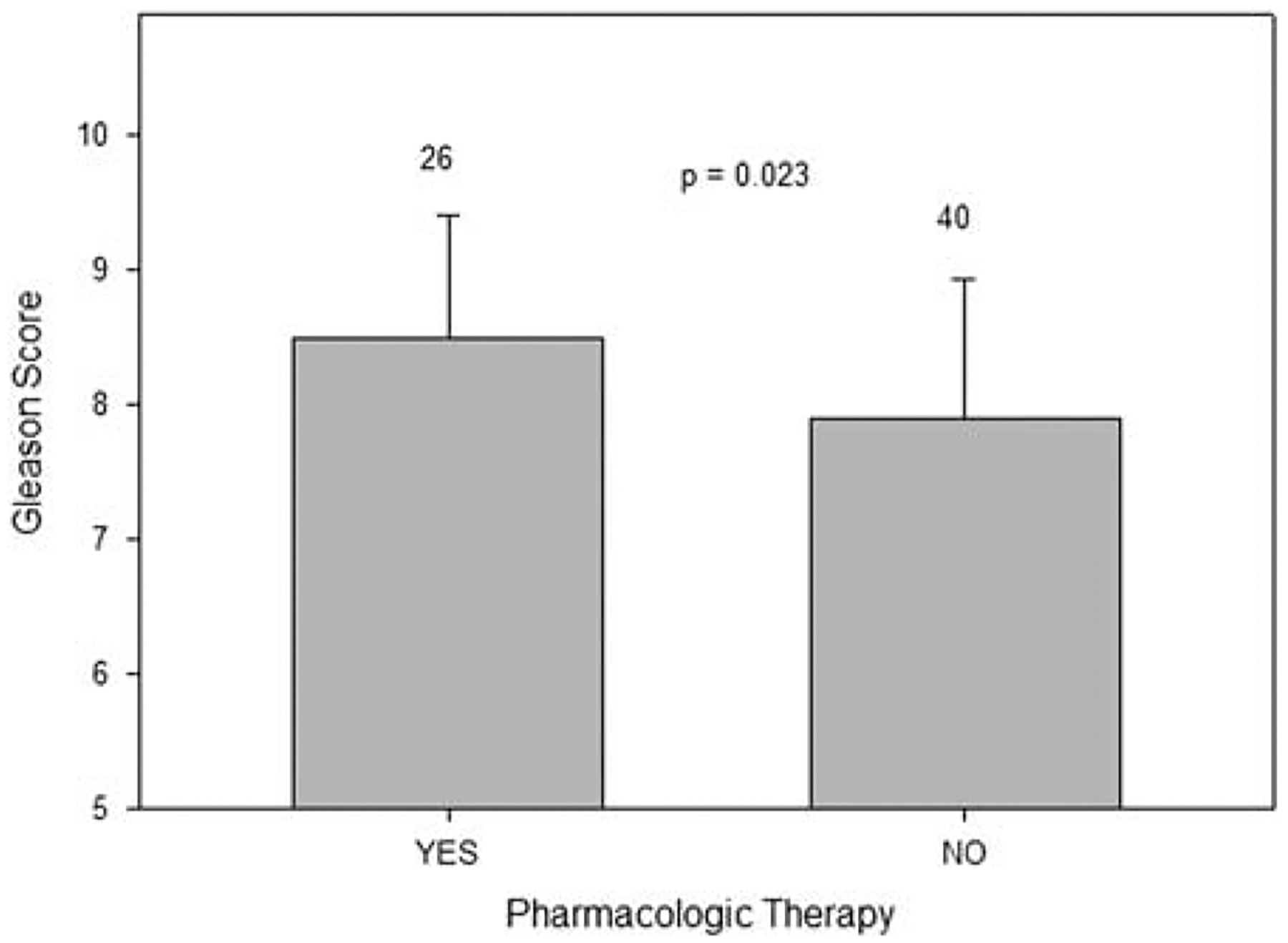
Patients with prostate cancer receiving pharmacologic therapy had a tumor with a significantly higher Gleason score (mean ± SD). Number of cases in each group is above the corresponding error bar.
BIN1 forms part of a network that interacts with the MYC oncogene (Fig. 8).
FIGURE 8.
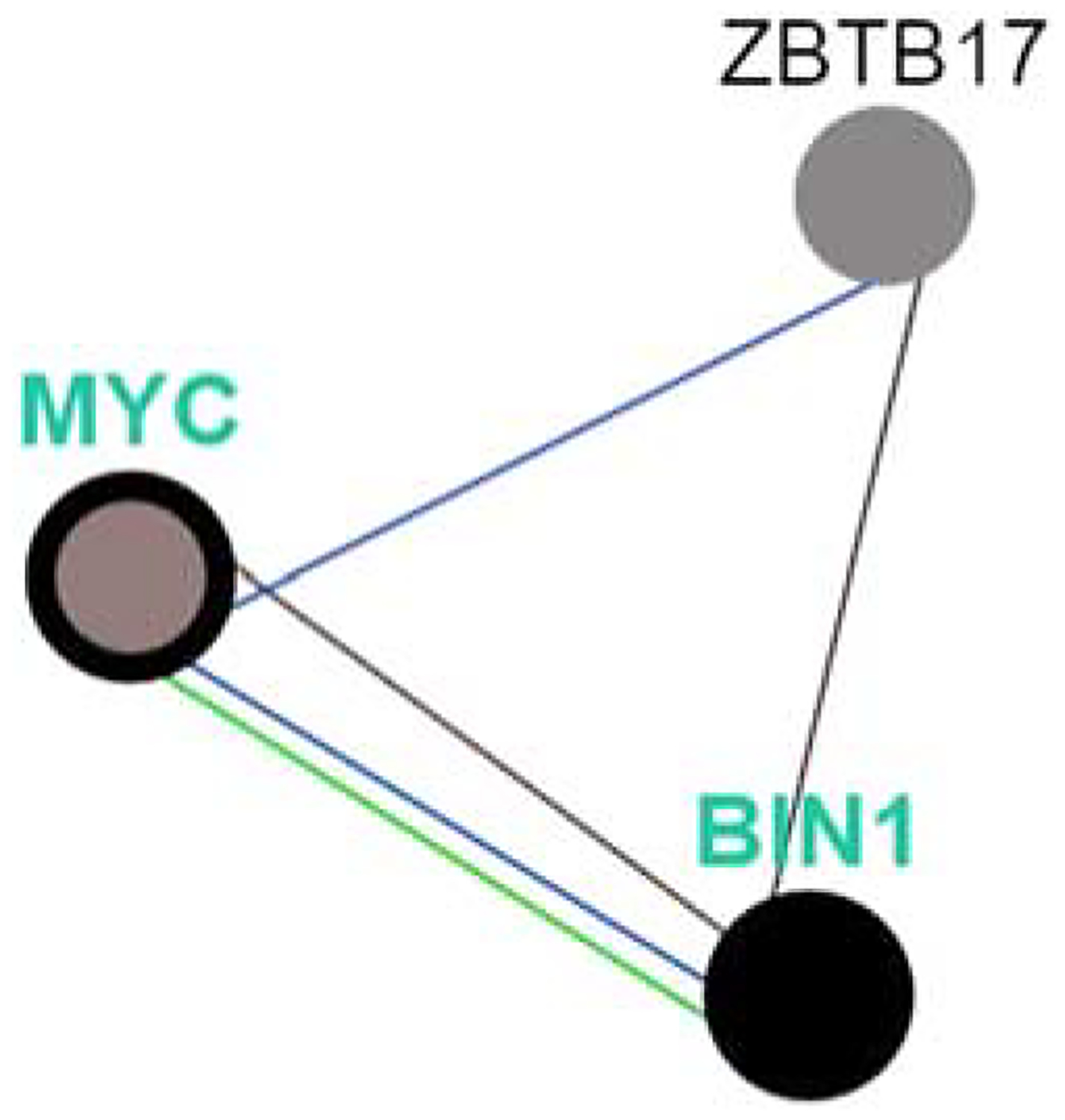
BIN1 forms part of a network that interacts with the MYC oncogene. MYC is altered in 8% of prostate cancers, BIN1 in 4%. Blue line: controls state of change. Green line: controls expression. Brown line: part of a complex. ZBTB17 encodes a zinc finger protein involved in the regulation of c-MYC (http://www.pathwaycommons.org/pcviz).
DISCUSSION
Biological processes or pathways in cancer are often deregulated through different genes or by multiple different mechanisms. But cancer gene alterations usually do not occur at random. Alterations of certain cancer genes tend to co-occur, indicating that they may work in tandem to drive tumor formation and development.18 This may be the case in prostate cancer with the co-occurring alterations shown in Table 1 of SPOP/BIN1 and SPTA1/CD2AP.
According to TCGA, SPOP is the third most mutated gene in prostate cancer, whereas SPTA1 is the eighth most mutated gene. SPOP mutation drives prostate tumorigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling.20 In one study of prostate cancer, SPTA1 and SPOP harbored mutations in 2 of 7 tumors. SPTA1 encodes a scaffold protein involved in erythroid cell shape specification, whereas SPOP encodes a modulator of Daxx-mediated ubiquitination and transcriptional regulation.21
SPOP is an upstream negative regulator for histone deacetylase 6 (HDAC6), which plays critical roles in human tumorigenesis and metastasis. HDAC6 stability and SPOP loss-of-function mutations might lead to elevated levels of the HDAC6 oncoprotein to facilitate tumorigenesis and metastasis in many human cancers.22 The E3 ubiquitin ligase CHIP interacts with HDAC6 and promotes its poly-ubiquitination to suppress abnormal accumulation of the microtubule-binding protein tau, which is correlated with cognitive decline in AD.23
Bridging integrator 1 (BIN1) is a widely expressed adaptor protein that is part of the BIN1/amphiphysin/RVS167 (BAR) family. A large genome-wide association study revealed that BIN1 is a risk factor for late-onset AD. This association was confirmed in subsequent genome-wide association studies in different populations and in meta-analyses. Genome-wide association studies have identified BIN1 within the second most significant susceptibility locus in late-onset AD.24
Dynamic regulation of the BIN1-Tau interaction is involved in AD and a high level of BIN1 expression may be protective,25 whereas BIN1 loss in AD allows phosphorylated tau to be mis-sorted to synapses which likely alters the integrity of the postsynapse, alongside reducing the functionally important release of physiological forms of tau.26
The observed BIN1 coexisting mutations and expression within prostate cancer tissue might simply be through BIN1’s known tumor suppressor effects.27 But our finding that BIN1 expression is inversely related to the Gleason score (Fig. 5) could suggest germline BIN1 alterations.
The MYC family consists of 3 related human genes; one is c-MYC. The N terminus of the c-MYC oncoprotein interacts with BIN1.28 MYC is activated at the earliest phases of prostate cancer and in its position on chr8q24 is linked to prostate cancer aggressiveness.29
CD2AP, a scaffolding molecule regulating signal transduction and cytoskeletal molecules, is implicated in AD pathogenesis. Several single nucleotide polymorphisms (SNPs) in CD2AP are associated with a higher risk for AD. mRNA levels of CD2AP are decreased in peripheral lymphocytes of patients with sporadic AD.30
In the largest study of ADT to date, 154,089 men with prostate cancer, those who underwent ADT had 14% or 20% greater chance of developing AD or dementia, respectively. Those who received ≤ 4 doses had a 19% chance of being diagnosed with either condition, whereas the 5 to 8 dose group reached 28% likelihood of AD and 24% chance of dementia. Eight or more doses and the chances were 24% and 21%, respectively. The study suggests an association between ADT and subsequent dementia but does not investigate possible biological mechanisms of the association. The authors conclude that clinicians need to carefully weigh the long-term risks and benefits of exposure to ADT in patients with a prolonged life expectancy and stratify patients on the basis of dementia risk before ADT initiation.9
ADT and testosterone deprivation may impair memory in older men,31 whereas testosterone supplementation can augment memory and spatial perception. Studies of prostate cancer demonstrate that androgen deprivation drugs adversely affect cognition,32 which returned to baseline when drugs were withdrawn.33
Alzheimer’s symptoms are usually preceded by a preclinical phase that may be 16 years long.34 We propose that the ADT dosage reflects the severity of a process that is already underway. The severity is determined by the genetics of the tumor itself, at least in part by the interactions of SPOP/BIN1, MYC/BIN1, and SPTA1/CD2AP. ADT is not causing new cases of AD. The oncologist treats higher-grade prostate cancer with more ADT (pharmacologic therapy, Fig. 7), which serves as a surrogate marker for disease severity. In the TCGA data, patients with a higher Gleason score were more likely to receive pharmacologic therapy.
A weakness in our study is that we present somatic mutation data rather than germline data. Coexisting germline mutations would be present in every cell of the body including the brain, where AD is manifest. A somatic mutation develops in a specific cell and is then propagated to daughter cells. In our study, the cell with the mutation is in the prostate. We are uncertain whether these same mutations coexist in the brain.
Nevertheless, our analysis of TCGA data does not support the idea that ADT causes AD or dementia in men with prostate cancer.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Zakaib D. Prostate cancer therapy makes dementia more likely. Alzforum. 2019. Available at: www.alzforum.org/news/research-news/prostate-cancer-therapy-makes-dementia-more-likely. Accessed November 20, 2019. [Google Scholar]
- 2.Alibhai SM, Timilshina N, Duff-Canning S, et al. Effects of long-term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer. 2017;123:237–244. [DOI] [PubMed] [Google Scholar]
- 3.Baik SH, Kury FSP, McDonald CJ. Risk of Alzheimer’s disease among senior medicare beneficiaries treated with androgen deprivation therapy for prostate cancer. J Clin Oncol. 2017;35:3401–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehrer S, Rheinstein PH, Rosenzweig KE. No relationship of antiandrogens to Alzheimer’s disease or cognitive disorder in the MedWatch database. J Alzheimers Dis Rep. 2018;2:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzouk S, Naglie G, Tomlinson G, et al. Impact of androgen deprivation therapy on self-reported cognitive function in men with prostate cancer. J Urol. 2018;200:327–334. [DOI] [PubMed] [Google Scholar]
- 6.Lehrer S. Editorial comment. J Urol. 2018;200:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nead KT, Gaskin G, Chester C, et al. Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol. 2016;34:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohile SG, Lacy M, Rodin M, et al. Cognitive effects of androgen deprivation therapy in an older cohort of men with prostate cancer. Crit Rev Oncol Hematol. 2010;75:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayadevappa R, Chhatre S, Malkowicz SB, et al. Association between androgen deprivation therapy use and diagnosis of dementia in men with prostate cancer. JAMA Netw Open. 2019;2: e196562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond J, Le Q, Goodyer C, et al. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. [DOI] [PubMed] [Google Scholar]
- 11.Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10:77–82. [DOI] [PubMed] [Google Scholar]
- 12.Rosario ER, Carroll JC, Oddo S, et al. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci. 2006;26:13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pike CJ, Carroll JC, Rosario ER, et al. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedge DC, Gundem G, Mitchell T, et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet. 2018;50:682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutter C, Zenklusen JC. The cancer genome atlas: creating lasting value beyond its data. Cell. 2018;173:283–285. [DOI] [PubMed] [Google Scholar]
- 16.Goldman M, Craft B, Swatloski T, et al. The UCSC Cancer Genomics Browser: update 2015. Nucleic Acids Res. 2015;43: D812–D817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahriyari L Effect of normalization methods on the performance of supervised learning algorithms applied to HTSeq-FPKM-UQ data sets: 7SK RNA expression as a predictor of survival in patients with colon adenocarcinoma. Brief Bioinform. 2019;20:985–994. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawy T. A pan-cancer atlas. Nat Methods. 2018;15:407. [DOI] [PubMed] [Google Scholar]
- 20.Blattner M, Liu D, Robinson BD, et al. SPOP mutation drives prostate tumorigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell. 2017;31:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan Y, Ci Y, Dai X, et al. Cullin 3SPOP ubiquitin E3 ligase promotes the poly-ubiquitination and degradation of HDAC6. Oncotarget. 2017;8:47890–47901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook C, Gendron TF, Scheffel K, et al. Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation. Hum Mol Genet. 2012;21:2936–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rossi P, Buggia-Prevot V, Clayton BL, et al. Predominant expression of Alzheimer’s disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Mol Neurodegener. 2016;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartori M, Mendes T, Desai S, et al. BIN1 recovers tauopathy-induced long-term memory deficits in mice and interacts with Tau through Thr (348) phosphorylation. Acta Neuropathol. 2019;138:631–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glennon EB, Lau DH-W, Gabriele RMC, et al. Loss of the Alzheimer’s-linked bridging integrator 1 (BIN1) protein affects synaptic structure and disrupts tau localisation and release. bioRxiv. 2019:646406. [Google Scholar]
- 27.Ge K, Minhas F, Duhadaway J, et al. Loss of heterozygosity and tumor suppressor activity of Bin1 in prostate carcinoma. Int J Cancer. 2000;86:155–161. [DOI] [PubMed] [Google Scholar]
- 28.Pineda-Lucena A, Ho CS, Mao DY, et al. A structure-based model of the c-Myc/Bin1 protein interaction shows alternative splicing of Bin1 and c-Myc phosphorylation are key binding determinants. J Mol Biol. 2005;351:182–194. [DOI] [PubMed] [Google Scholar]
- 29.Koh CM, Bieberich CJ, Dang CV, et al. MYC and prostate cancer. Genes Cancer. 2010;1:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao QQ, Chen YC, Wu ZY. The role of CD2AP in the pathogenesis of Alzheimer’s disease. Aging Dis. 2019;10:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bussiere JR, Beer TM, Neiss MB, et al. Androgen deprivation impairs memory in older men. Behav Neurosci. 2005;119:1429–1437. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez BD, Jim HSL, Booth-Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33:2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019;25:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


