Abstract
Objective:
The contributions of intervertebral disc disease and subject-specific covariates to systemic inflammation in low back pain are unknown. We examined the effects of symptomatic disc herniation (DH) and MRI herniation severity on serum cytokine levels in clinical subjects.
Design:
Cytokine levels from lumbar DH subjects (N=78) were compared to control subjects (N=57) accounting for effects of DH, age, BMI and gender. Effect of DH severity on cytokine levels was analyzed on subsets of subjects with acute or chronic pain. Serum cytokines were also analyzed in a subset of patients between pre- and 3 months post-surgery.
Results:
Cytokine levels were elevated in the serum of patients with symptomatic DH, and the covariates age, BMI and gender significantly contributed to levels of some cytokines. Severity of herniation was a significant contributor to pain intensity (VAS), serum levels of HMGB1, PDGFbb, and IL-9. The relationship between DH severity and cytokine levels was confirmed in subjects with chronic, but not acute symptoms. Serum levels of MIF decreased, whereas levels of CCL3, CCL11, CXCL1, and CXCL10 were significantly elevated post surgery.
Conclusions:
This study is the first to show that DH severity is coordinately associated with changes in serum levels of inflammatory cytokines in chronic pain subjects. HMGB1, PDGFbb and IL-9 are novel mediators of increasing DH severity, indicative of cellular damage, neuro-inflammation and angiogenesis. Resolution of inflammation was observed with decrease in MIF post surgery. However, elevated chemokine levels indicate ongoing remodeling and wound healing at 3-month time point.
Keywords: Intervertebral disc, inflammation, herniated disc, MRI severity, pain
Introduction
Back pain is the leading cause of disability worldwide, creating a significant burden on patients and the economy (1). One of the most prevalent disc pathologies that results in lumbar radicular pain is disc herniation (DH), defined as the displacement of intervertebral disc (IVD) material beyond the normal margins of the disc space. Degradation of the IVD is mediated by many biochemical factors; catabolic enzymes such as matrix metalloproteases (MMPs) directly act on the extracellular matrix promoting remodeling and degradation, whereas pro-inflammatory cytokines increase the levels and/or activity of catabolic enzymes (2, 3).
DH is both a mechanical and biochemical process, where disc contact with spinal nerves causes compression, chemical irritation, inflammation, and pain (4, 5). Herniated disc tissue exhibit an inflammatory cell infiltration and increased pro-inflammatory cytokines (6-9). Pro-inflammatory cytokines lower the threshold for pain (10) and increase pain sensitivity (11). In animal injury models, stimulating nerve roots in vivo with pro-inflammatory cytokines or with autologous NP tissue produces a neuropathological behavior, that is mitigated when the pro-inflammatory cytokine or signaling is neutralized (12-15). Despite the evidence that behavioral hypersensitivity is associated with dynamic inflammation in response to induced injury, studies in patient populations represent complex interactions between disease states, subject-specific covariates and time. Pain is a dynamic and subjective experience, and the use of biochemical markers to pinpoint molecular origin of pain or to drive personalized treatment could be clinically impactful.
Increasing evidence suggests that inflammatory mediators can be measured systemically (16-20), where serum levels of IL-2, IL-6, IL-8, TNF-α and others have been shown to be elevated in DH patients (17, 19). Chronic lumbar radicular pain is also associated with a persistent increase of serum IL-6 and IL-8 after DH (21). A profiling study found that significant differences exist between those with chronic painful DH and the patients who recovered from DH (22). Despite evidence implicating DH in systemic inflammation, other subject-specific factors such as age, BMI and gender may contribute to inflammatory levels (23, 24). Yet such covariate factors are not typically accounted for in studies analyzing inflammatory cytokine levels in patients with LBP due to DH or other pathologies. Studies show associations between IL-6 levels and BMI, symptom duration, and age, while MMP-1 levels were associated with age (21, 25, 26). Importantly, the relationship between serum cytokine profiles and structural indicators of spine disease, as diagnosed using radiological examination are largely unknown.
In this study, we measured circulating cytokines in patients with symptomatic DH compared to a control cohort, accounting for effects of age, BMI and gender. We also evaluated the relationship between inflammatory profiles and structural indications of disease based on magnetic resonance imaging, the gold standard radiological exam for diagnoses of DH. We specifically compared cytokine levels with severity of herniation, using a measure of spinal canal intrusion. In a subgroup analysis, we compare cytokine profiles in patients from pre- to post- surgical treatment, to determine if surgery provides an opportunity for normalizing aberrant cytokine profiles in DH patients. Our findings address a critical translational gap in the immune response to DH, and to advance the relevance of serum cytokine as potential biomarkers of painful DH pathology.
Methods
DH Subjects
This study (NCT01633034) was approved by the Institutional Review Board of Northwell Health. Subjects with DH were recruited from a spine neurosurgery practice (N=78). Written informed consent was obtained from all subjects prior to enrollment. Study inclusion criteria included patients 18 years of age or older, requiring surgery in the lumbar spine (L1-L2 to L5-S1) for treatment of a DH. Procedures including lumbar fusion, discectomy, laminectomy and/or minimally invasive discectomy were recruited. Subjects requiring revision surgery in the lumbar spine, had a history of prior history of discography procedures, or known inflammatory conditions (e.g. rheumatoid arthritis, systemic lupus erythematosus, gout, osteomyelitis, other infections) or history of cancer were excluded.
Clinical data including basic demographic information (age, gender, BMI), underlying diagnosis, prior treatment history, and surgical details were collected. Patient reported outcomes were also evaluated prior to surgery. Pain intensity was evaluated using a 10-point visual analog scale (VAS). Duration of symptoms was collected and categorized into acute (i.e. <6 months) or chronic pain (>6 months).
Control Subjects:
Control subjects, with no history of LBP, were also recruited using advertisements (N=57). Written informed consent was obtained from control subjects prior to recruitment. Subjects 18 years of age and older with no history of spinal injury or spine surgery were included in the control cohort. Venipuncture was performed to collect serum using same methods as described for clinical subjects. Demographic information (age, gender, BMI) and medical history were collected from each subject.
Radiological Analysis:
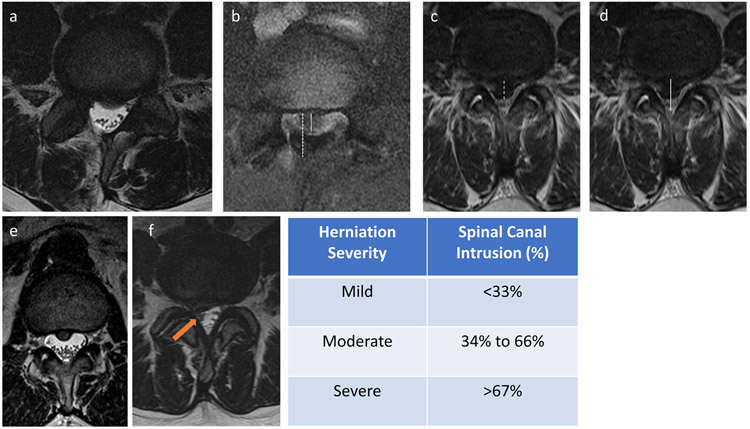
Pre-operative MRI scans of DH subjects were performed on a 3.0T MR scanner using a multitransmit (parallel transmission) 3.0T Philips Achieva TX MR system (Philips Healthcare) and a 16-channel SENSE spine coil (Invivo Corporation). Axial and sagittal T1- and T2- weighted clinical MRI scans were evaluated on two different days for degree of herniation by a board-certified neuro-radiologist (CP). Measurements of each subject’s images were made on separate days, and the resulting intra-rater reliability was found to be 78% based on N=80 images. The herniation size and central spinal canal diameter were measured in the anterior-posterior dimension (Figure 1). Herniation severity was performed to determine the spinal canal intrusion (%), computed from the ratio of mean herniation size relative to mean canal size. Canal intrusion of less than one third was considered ‘mild’, between one and two-thirds as ‘moderate’ and greater than two-thirds as ‘severe’. The presence of nerve or nerve root compression or involvement in the DH was also noted and categorized (yes/no). Overall disc integrity was also evaluated and graded using Pfirrmann grade, on a scale of I to V, where I represents a healthy disc and V represents severe disc degeneration (27).
Figure 1.
Radiological analysis of herniation severity. (a,b) moderate-sized herniation showing (a) T2-weighted MRI and (b) measurement of disc herniation (dashed line) and the central canal diameter (solid line) in the anterior to posterior dimension. (c,d) Large (i.e. severe) central disc herniation showing measurements of disc herniation (dashed) and the central spinal canal (solid line) in the anterior to posterior dimensions. (e) Axial T2 weighted image of a small (i.e. mild) central herniation with ventral effacement of the thecal sac but no associated nerve root compression. (f) Right subarticular herniation with ventral effacement of the thecal sac and compression of the traversing nerve root.
Blood sample collection:
Blood samples were obtained from each DH subject on day of surgery before administration of anesthesia and the start of any surgical procedures. One 10cc sample of whole blood was collected via venipuncture (BD Vacutainer 367820) and maintained at 2-8°C until processing. For each subject, blood sample was centrifuged to isolate serum. Serum was aliquoted, and stored at −80°C.
Pre to Post Surgery Analysis:
A small cohort of DH subjects (N=5), which were initially evaluated at baseline (pre-treatment), were re-evaluated at 3 months post surgery (post-treatment). At the post-treatment time point, a blood sample were obtained from each subject at the time of their office visit with the treating surgeon, using the same approach described above. Serum was aliquoted, and stored at −80°C until biochemical analysis.
Biochemical analysis:
All measurements and analyses were performed on coded samples. Serum samples were analyzed for (a) cytokine profiles using a multiplex immune-bead assay (Bio-Plex Pro Human Cytokine Grp 1 Panel 27-plex, BioRad), (b) MMP levels using an electrochemiluminescent immunoassay (Human MMP 3-plex UltraSensitive Kit, MesoScale Discoveries), and (c) High mobility group box 1 (HMGB1) using a commercial ELISA (IBL international). The assays were performed according to manufacturers’ protocols. For the cytokine multiplex samples were diluted 1:4 and were assayed in duplicate to measure interleukin (IL)-10, IL-12p70, IL-13, IL-15, IL-17, IL-1β, IL-1Rα, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, Eotaxin, Fibroblast growth factor basic (FGF-β), granulocyte colony stimulating factor (G-CSF), Granulocyte macrophage (GM)-CSF, Interferon gamma (IFN-γ), Interferon gamma-induced protein 10 (IP-10/CXCL10), C-C Motif Chemokine Ligand (CCL) −2 (CCL2/MCP-1), CCL3/MIP1α, CCL4/MIP1b, platelet-derived growth factor (PDGFββ), Regulated on activation normal T cell expressed and secreted (RANTES/CCL5), Tumor necrosis factor alpha (TNF-α), Vascular endothelial growth factor (VEGF). For the MMP assay, samples were diluted 1:10 and were assayed in duplicate to measures MMP-1, MMP-3, and MMP-9 levels. For measuring HMGB1 levels, serum samples were assayed undiluted. The lower limits of detection (LLOD) were noted for each analyte and plate, and any sample that yielded a below detection result was imputed the LLOD for that cytokine. We measured multiple biochemical factors from the same samples, but chose to not correct for multiple comparisons across the different analytes because this would enhance the probability of type II errors (28).
Statistical Analysis:
All analyses were performed on coded data sets using STATISTICA (Version 13.3, TIBCO Software). In all tests, p<0.05 was considered significant.
Comparing control vs. DH cohorts:
Age, gender and BMI were compared between control and DH cohorts using the non-parametric Mann Whitney U test. A multivariate regression analysis was performed where diagnosis (DH, control) and gender (male, female) were modeled as categorical predictors and age and BMI were modeled as continuous predictors of each biochemical mediator. The t-statistic and p-value are reported, as well as beta (β) coefficient and standard error of β.
Effect of DH severity on biochemical levels:
Age, gender, BMI and symptom duration were compared across the DH severity groups (mild, moderate, severe) using Kruskal Wallis ANOVA. Biochemical levels within the DH cohort were analyzed for their relationship with herniation severity (mild, moderate, severe) or Pfirmann grade (Grades II, III, IV, V) using a non-parametric Kruskal Wallis ANOVA and multiple comparisons were made with a Bonferroni adjustment. For analyzing the effect of DH, a sample size of 15 subjects per group affords >80% power to detect differences in cytokine levels (e.g. IL-6) based on prior studies (25); increasing the sample size to 33 subjects per group increases power to >95%. The root mean square standardized effect (RMSSE) size was also computed using 1-way ANOVA. The relationship between cytokine levels and spinal canal intrusion (%) or VAS scores was assessed using Spearman correlations. Radiographic features such as presence of nerve root involvement (Yes, No) were examined for their effect of biochemical factors using Mann Whitney U test.
Profiles in acute or chronic pain:
Separate analyses of DH subjects were performed for those with acute (< 6 months, N=33) or chronic symptoms (> 6months, N=36). Biochemical levels (N=69) and VAS (N=34) were analyzed for their relationship with herniation severity (mild, moderate, severe) using a non-parametric Kruskal Wallis ANOVA and multiple comparisons were made with a Bonferroni adjustment. The relationship between cytokine levels and VAS scores in acute or chronic subgroups were assessed using Spearman correlations.
Biochemical factors profiles from pre to post treatment:
To compare cytokine levels in subjects over time from pre- to post- treatment, cytokine levels were analyzed using a paired Wilcoxon singed rank test.
Results
Subject Characteristics
Overall, this study recruited 135 subjects with an average age of 44 years, and a gender distribution of 60% female (N=81) / 40% male (N=54). Demographics (age, gender, BMI) of the control and DH groups are presented in Table 1. When comparing the control and DH cohorts, there were significant differences in the mean age (Control: 39.5, DH: 47.3, p=0.000997), a trend for differences in BMI (control: 26.9, DH: 28.6, p=0.07), but no significant difference in gender distribution (p=0.95) (Table 1).
Table 1.
Summary of demographics of control and DH cohorts (top) along with summary of demographics of DH subjects by herniation severity (bottom)
| Control | DH | p-value | Total | |
|---|---|---|---|---|
| Number of Subjects | 57 | 78 | 135 | |
| Gender Distribution | ||||
| Male: N (%) | 23 (40%) | 31 (40%) | 0.9456 | 54 (40%) |
| Female: N (%) | 34 (60%) | 47 (60%) | 81 (60%) | |
| Age (mean ± SD) | 39.5 ± 13.3 | 47 ± 13.4 | 0.000997 | |
| BMI (mean ± SD) | 26 ± 5.3 | 28.6 ± 5.6 | 0.0688 | |
| Mild | Moderate | Severe | p-value | |
| N | 30 | 33 | 15 | |
| Age | 48 ± 2.1 | 49 ± 2.3 | 41± 4.3 | 0.146 |
| BMI | 29.3 ± 1.0 | 28.9 ± 0.99 | 26.5 ± 1.3 | 0.177 |
| Gender Distribution | ||||
| Male: N (%) | 12 (40%) | 12 (36%) | 7 (46%) | 0.798 |
| Female: N (%) | 18 (60%) | 21 (64%) | 8 (54%) | |
Within the DH cohort, subjects were categorized by herniation severity into mild, moderate and severe groups. There was no significant difference in age (p=0.15), gender (p=0.80) or BMI (p=0.18) between DH severity groups (Table 1). We observed presence of a sequestered DH in 15% of subjects (N=12/78), confirmed radiologic nerve involvement in 68% (N=53/78), evidence of spinal stenosis in 62% (N=48/78), and low prevalence of listhesis (8%). In the Pfirmann grading analysis, grades ranged from II to V with the following breakdown (N=8 Grade II, N=31 Grade III, N=31 Grade IV, N=7 Grade V). The median patient reported VAS was 7 (IQR: 5-8, N=34). The prevalence of patients reporting acute (48%, N=33/69) versus chronic (52%, N=36/69) symptoms were similar.
Biochemical factors in DH and control cohorts
Multivariate regression analysis found that DH, age, gender and BMI were significant predictors of certain biochemical levels (Table S1). Table 2 summarizes the effects of diagnosis, where DH was found to be a significant contributor to the levels of 21 mediators. Levels of following biochemical factors were significantly greater in DH compared to control subjects (fold changes shown in table 2): CCL2/MCP-1, CCL4, CCL5/RANTES, FGF basic, G-CSF, GM-CSF, HMGB1, IFN-g, IL-1b, IL-1ra, IL-2, IL-5, IL-6, IL-7, IL-9, IL-12(p70), IL-15, IL-17, MMP-9, PDGF-bb, and VEGF. CCL3/MIP1a, CCL11/Eotaxin, CXCL10, IL-4, IL-8, IL-10, IL-13, MMP-1, MMP-3, and TNF-α did not exhibit a significant contribution of DH on levels in the multivariate regression. Age was a significant predictor of Eotaxin (p=0.0003), IL-13 (p =0.037), IL-17 (p=0.0082), IL-1b (p=0.033), IL-4 (p= 0.0004), CXCL10/IP-10 (p= 0.044), and PDGF-bb (p=0.025), and BMI was a significant predictor of IL-1ra (p=0.0014). Gender was a significant predictor of IL-17 levels (Female: 12.18 ± 0.58 pg/ml; Male: 15.19 ± 1.41 pg/ml; p=0.045) and MMP-3 (Female: 12194 ± 950 pg/ml; Male: 25027 ± 4269 pg/ml; p=0.0011), with higher levels of both factors in males.
Table 2:
Effect of Diagnosis on Cytokine Levels- Mean levels and standard errors of biochemical factors measured in serum of control and DH subjects. Analysis was performed using regression model where cytokine levels were analyzed as a function of group (DH vs control), age, gender and BMI. p-value is shown for diagnosis (group) predictor. *denotes analytes that had at least 20% of samples from DH and control groups below LLOD. ^ denotes analytes that had at least 20% of control group samples below LLOD.
| Cytokine | Control Mean |
Control Std. Err |
DH Mean | DH Std. Err |
Fold Change (DH/Control) |
p-value |
|---|---|---|---|---|---|---|
| CCL11 / Eotaxin | 120.4 | 6.7 | 125.9 | 7.6 | 1.1 | 8.10E-01 |
| CCL2 / MCP-1 | 95.1 | 5.0 | 193.5 | 13.4 | 2.0 | 2.60E-08 |
| CCL3 / MIP-1a | 2.5 | 1.8 | 0.9 | 0.1 | 0.4 | 3.50E-01 |
| CCL4 / MIP-1b | 104.6 | 1.7 | 117.7 | 2.4 | 1.1 | 1.00E-04 |
| CCL5 / RANTES | 4396.2 | 209.1 | 9117.8 | 720.6 | 2.1 | 6.30E-07 |
| CXCL10 / IP-10 | 159.3 | 11.8 | 151.3 | 10.4 | 1.0 | 2.20E-01 |
| FGF basic ^ | 2.2 | 0.1 | 8.2 | 1.5 | 3.8 | 1.40E-03 |
| G-CSF * | 3.7 | 0.5 | 7.9 | 0.8 | 2.1 | 4.00E-04 |
| GM-CSF ^ | 0.6 | 0.0 | 2.6 | 0.2 | 4.2 | 1.80E-13 |
| HMGB1 | 3.4 | 0.5 | 6.5 | 0.6 | 2.0 | 4.40E-05 |
| IFN-g | 1.7 | 0.1 | 4.9 | 0.5 | 3.0 | 1.80E-05 |
| IL-10 * | 1.4 | 0.0 | 1.5 | 0.1 | 1.1 | 4.20E-01 |
| IL-12(p70) * | 1.8 | 0.0 | 3.5 | 0.5 | 1.9 | 3.10E-02 |
| IL-13 * | 0.3 | 0.0 | 0.5 | 0.1 | 1.5 | 5.40E-02 |
| IL-15 * | 7.6 | 0.4 | 12.0 | 0.8 | 1.6 | 8.70E-06 |
| IL-17 | 10.8 | 0.4 | 15.3 | 1.1 | 1.4 | 5.20E-03 |
| IL-1b ^ | 0.2 | 0.0 | 0.6 | 0.1 | 2.6 | 1.10E-02 |
| IL-1ra | 69.0 | 5.5 | 189.2 | 17.6 | 2.7 | 4.40E-07 |
| IL-2 ^ | 0.8 | 0.1 | 2.3 | 0.1 | 2.9 | 1.10E-16 |
| IL-4 | 1.5 | 0.1 | 1.8 | 0.1 | 1.2 | 1.40E-01 |
| IL-5 ^ | 0.6 | 0.1 | 3.1 | 0.3 | 4.9 | 3.10E-11 |
| IL-6 | 1.4 | 0.1 | 3.8 | 0.3 | 2.8 | 2.40E-08 |
| IL-7 ^ | 0.6 | 0.1 | 1.7 | 0.2 | 2.9 | 1.70E-07 |
| IL-8 | 13.9 | 3.4 | 12.5 | 2.0 | 0.9 | 7.10E-01 |
| IL-9 | 15.9 | 0.4 | 23.7 | 1.6 | 1.5 | 4.00E-04 |
| MMP-1 | 15558.7 | 1690.4 | 18245.7 | 1469.4 | 1.2 | 3.80E-01 |
| MMP-3 | 13893.6 | 968.3 | 19836.8 | 3139.6 | 1.4 | 1.90E-01 |
| MMP-9 | 161482.2 | 8754.3 | 210786.3 | 20642.0 | 1.3 | 2.80E-02 |
| PDGF-bb | 764.9 | 34.0 | 1401.6 | 76.2 | 1.8 | 5.30E-11 |
| TNF-a | 21.3 | 0.7 | 29.5 | 3.5 | 1.4 | 2.60E-01 |
| VEGF ^ | 3.2 | 0.3 | 15.0 | 2.0 | 4.7 | 8.40E-06 |
Relationship between radiological indicators and biochemical factors
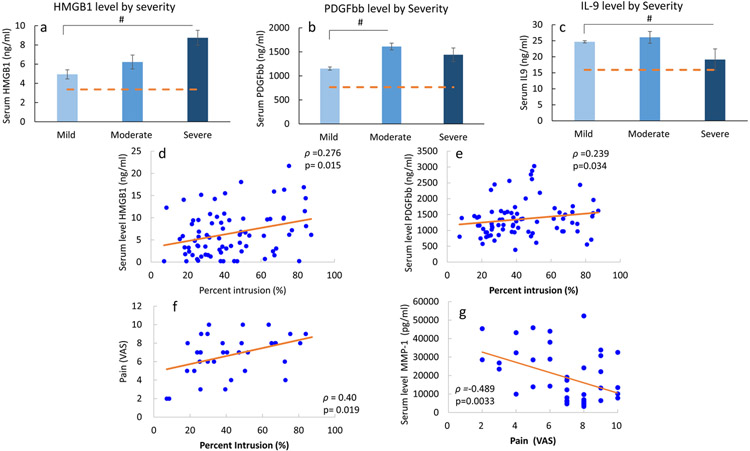
We investigated the effects of herniation severity on serum levels in the DH cohort. Herniation severity had a significant effect on serum levels of HMGB1 (p=0.018, RMSSE=0.91), IL-9 (p=0.035, RMSSE=0.99), and PDFGbb (p=0.041, RMSSE=0.72) (Figure 2). In severe herniation, serum HMGB1 (p=0.014) and IL-9 (p=0.029) levels were significantly greater than in subjects with mild herniation. Serum PDGFbb was significantly higher in subjects with moderate compared to mild herniation (p=0.049). Serum HMGB1 and PDGFbb levels were also positively correlated with spinal canal intrusion (%) with a Spearman correlation coefficient (rho) of 0.28 (p=0.015) and 0.24 (p=0.034), respectively (Figure 2). Nerve root involvement was not found to be a significant contributor to the serum cytokine levels. When analyzing cytokine levels as a function of overall disc integrity, Pfirmann grade was not a statistically significant contributor to cytokine levels. However, we noted a trend, in that Pfirmann grade contributed to serum levels of PDGFbb (p=0.051).
Figure 2.
Serum levels of (a) HMGB1, (b) PDGFbb, and (c) IL-9 in DH subjects as function of DH severity. #p<0.05 in indicated group vs. mild. Bar graphs represent mean and standard deviation, with dashed line representing mean level in control cohort. Spearman correlation coefficient (ρ) and p value for significant correlations observed % intrusion (of herniation) and serum levels of (d) HMGB-1 (N=78), (e) PDGF-bb (N=78), and (f) Pain (VAS; N=34). (g) Spearman correlation between VAS and MMP-1 levels (N=34). Each point on scatter plot represents one subject, and line represents the correlation fit.
Effect of pain intensity and symptom duration on biochemical factor levels
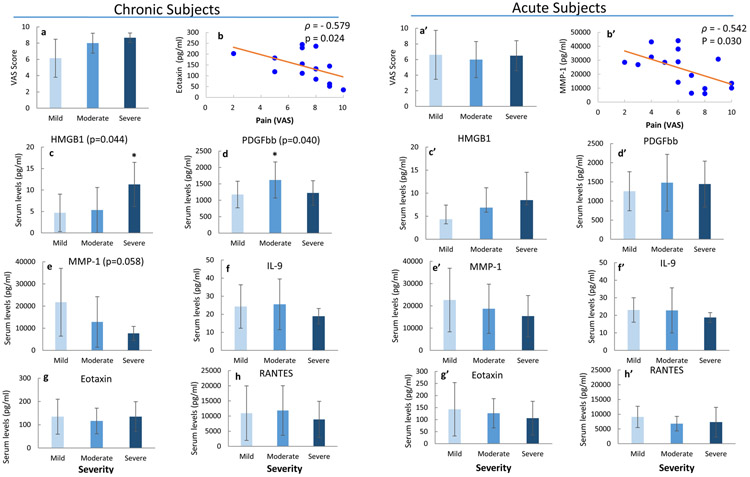
In all DH subjects, VAS score (i.e. pain) was positively correlated with spinal canal intrusion (rho=0.399, p=0.019), and MMP-1 levels were inversely correlated with VAS score (rho=−0.489, p=0.0033, Figure 2). However, VAS score was not correlated with levels of HMGB1, PDGFbb, IL-9 or other cytokines across all DH subjects. When assessing DH severity effects on biochemical factor levels in DH subjects based on symptom durations, we observed different relationship in the acute vs. chronic subgroups (Figure 3). In chronic subjects, pain (VAS) score was inversely correlated with serum Eotaxin levels (rho=−0.579, p=0.024). Levels of HMGB1 and PDGFbb significantly increased with DH severity (Figure 3c,d) in chronic pain group, while MMP1 levels exhibited a decreasing trend with increasing severity (p=0.058, Figure 3e). In acute pain subjects, VAS score was inversely correlated with MMP-1 levels (rho=−0.542, p=0.03, Figure 3b’). Other biochemical factors did not exhibit significant dependence on DH severity or pain in the acute subgroup.
Figure 3.
Effects of DH severity in chronic (a-h) and acute (a’-h’) subjects. (a, a’) VAS scores across severity groups. (b,b’) Spearman correlation between VAS and (b) Eotaxin or (b’) MMP-1 (ρ: Spearman correlation coefficient). Serum levels of (c,c’) HMGB1, (d,d’) PDGFbb, (e,e’) MMP-1, (f,f’) IL-9, (g,g’) Eotaxin, and (h,h’) RANTES in chronic and acute subjects, respectively. Bar graph are indicative of mean and standard deviation. * p<0.05 vs. Mild in multiple group comparison based on post-hoc test.
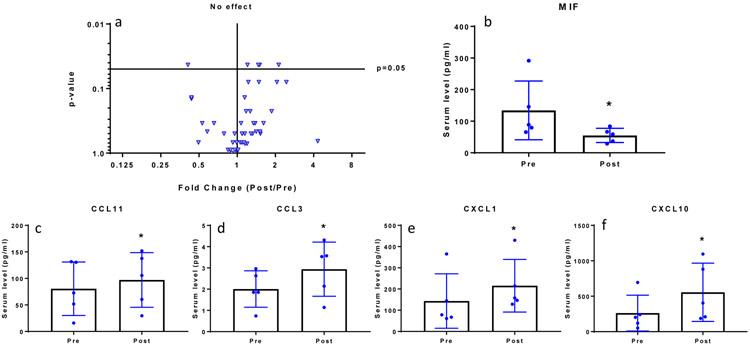
Biochemical factors profiles from pre to post treatment
Clinical and demographic variables of these subjects are described in Table 3. Differences in biochemical levels were observed between pre- and post-treatment are presented using a volcano plot (Figure 4). The x-axis represents fold change ratio in post-surgery levels normalized to pre-surgery levels, and the y-axis displays the p-value for the comparison. We found 5 mediators to be significantly different within the same cohort of subjects when evaluated at 3 months post surgery compared to pre-surgery (baseline). Serum levels of MIF significantly decreased post surgery compared to pre-surgery (p<0.05, Figure 4). Serum levels of Eotaxin (CCL11), MIP1a (CCL3), GROα (CXCL1), and IP10 (CXCL10) all significantly increased post surgery compared to pre surgery (p<0.05, Figure 4).
Table 3:
Patient demographic of cohort that was analyzed longitudinally from pre- to post-surgery.
| Subject ID |
Age | Gender | BMI | Pain Duration |
Spinal Level |
% intrusion |
DH Severity |
Pfirrmann Grade |
Nerve Root Involvement |
Sequest- ration |
Listhesis | Stenosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 163 | 66 | M | 25.8 | -- | L4-L5 | 36% | Moderate | IV | N | Y | Y | Y |
| 164 | 45 | F | 37.1 | acute | L5-S1 | 30% | Mild | V | Y | Y | Y | Y |
| 171 | 45 | M | 24.7 | acute | L5-S1 | 50% | Moderate | III | Y | N | N | N |
| 173 | 69 | F | 34.2 | chronic | L4-L5 | 26% | Mild | V | N | N | N | Y |
| 179 | 47 | M | 26.6 | chronic | L4-L5 | 45% | Moderate | IV | Y | N | N | N |
Figure 4.
(a) Volcano plot of serum cytokine levels post surgery vs. pre-surgery. Each triangle represents fold change (Post/Pre) in cytokine level (x-axis) and p-value (y-axis). Fold change=1 is indicated as ‘No effect’ and significance was set at p=0.05. Serum levels of (b) MF decreased post surgery, while levels of (c) CCL11, (d) CCL3, (e) CXCL1, and (f) CXCL10 significantly increased post surgery. In (b) to (f) scatter plot indicate individual patient levels pre- and post-surgery, with bar graph indicative of mean and standard deviation.
Discussion
The goal of this study was to examine the relationship between systemic levels of inflammatory mediators and IVD herniation, to identify the effects of herniation severity, pain intensity and duration of symptoms. Our findings provide evidence that cytokine levels are elevated in the serum of patients with DH and that the subject-specific covariates age, BMI and gender are significant contributors to some cytokines. Of the 21 factors we found elevated in DH subjects, only 3 mediators (HMGB1, PDGFbb and IL-9) were further dependent on herniation severity. Percent intrusion was also positively correlated with pain. Interestingly, these significant relationships between DH severity and cytokine levels were present in subjects with chronic symptoms, but did not hold for those with acute symptoms. Not surprisingly, we did not observe significant differences in serum cytokines with respect to disease severity as classified by Pfirrmann grade, consistent with prior findings (25, 29). Indeed, MRI signal grading of degeneration does not correlate to functional impairment or pain (30). These findings suggest that DH severity is a more sensitive indicator of mechanical disc injury than Pfirmann grade (31). We also observed resolution of inflammation mediated by MIF and that chemokines levels (CCL11, CCL3, CXCL1 and CXCL10) increased in patients at 3 months post surgery.
HMGB1 has been observed to mediate inflammation in multiple injury models. HMGB1, which acts a danger associated molecular pattern (DAMP), is released by injured cells as part of a response to tissue damage and inflammation (32, 33). Our findings indicate that increase herniation severity (as a mode of injury) results in corresponding increase in DAMP signal, presumably to alert the immune system of local tissue damage at site of herniation. HMGB1 is a potent pro-inflammatory mediator in degenerated human discs (33, 34), where IL-1β and HMGB1 additively promote the release of inflammatory cytokines and MMP expression. In the present study, HMGB1 was found to be a key factor related to DH, where serum levels were modulated by DH severity, particularly in patients with chronic herniation (>6 months). We postulate that increasing systemic levels of HMGB1 may be due to increasing levels of cellular damage and inflammation at the site of herniation compression. HMGB1 was also correlated with degree of intrusion into the spinal canal, which in turn was correlated with pain intensity (VAS). These findings suggest that elevated systemic levels of HMGB1 may be mediating a chronic compression injury response. Interestingly, HMGB1 has been implicated in the induction and resolution of pain behavior in rodent injury models (12, 35). The findings of the current study are the first to support the notion that perhaps neutralizing HMGB1 or blocking signaling mediated by HMGB1 may warrant consideration as a therapeutic intervention in severe chronic DH.
In the context of the IVD, PDGFbb enhances cell proliferation, differentiation, ECM expression, and reduces nucleus pulposus cell apoptosis (36, 37). In disc degeneration, PDGFbb levels are elevated, in part due to vascular ingrowth, causing increased cell proliferation and cell clustering, which may represent a tissue repair mechanism (38). The findings that serum PDGFbb levels increase with chronic DH severity may represent an upregulation of a repair mechanism in response to injury and inflammation, however it is unclear if elevated levels are reflective of an attempt to resolve the herniated disc. While recombinant PDGFbb has been proposed as potential therapeutic for disc disease (39), the findings of the current study may complicate the therapeutic relevance of recombinant PDGFbb for chronic DH.
Healthy disc tissue lacks vasculature and is considered to be an immune-privileged organ. Herniation of disc tissue into the epidural space evokes an autoimmune reaction that leads to the infiltration of immune cells which will interact with disc cells and secrete a variety of autoimmune related signaling molecules (40). In the current study, we found that IL-9 levels, which is thought to be a major effector cytokine of autoimmune disorders, is upregulated systemically in DH patients. Interestingly, IL-9, a pleiotropic cytokine that is released by activated Th9 cells, has been studied in a single study to our knowledge, and found to be increased in human degenerated disc tissues, though specific cellular contributions were not confirmed (41). IL-9 also promotes inflammatory pathogenesis of the IVD through TNF-α interactions (41). This is a highly novel candidate marker of disease severity and motivates the need for further investigations into the relevance of IL-9 and Th9 cells in DH.
Interestingly, we observed inverse relationship between pain intensity (VAS) and cytokine levels. In acute subjects, MMP-1 levels were inversely correlated with pain. MMP-1 (collagenase) promotes matrix breakdown and is involved in leukocyte trafficking; this inverse relationship may be a consequence of poor or ineffective matrix remodeling of the herniated tissue, leading to more pain. Interestingly, patients with chronic symptoms exhibited an inverse relationship between Eotaxin/CCL11 and pain levels. A prior study found that chronic stress and anxiety to be correlated with lower levels of CCL11 in healthy adults (42), suggesting perhaps that lower levels of CCL11 in chronic DH patients may be related to chronic stress or anxiety. These findings suggest that pain and associated stress are associated with suppression of some immune factors, which differed with symptom duration. Overall, our findings suggest that systemic inflammatory profiles vary between DH subjects with acute or chronic pain. When evaluating DH severity vs cytokines in acute subjects, we didn’t observe the same relationships as seen in all DH subjects. Indeed, our findings show that systemic inflammatory profiles have stronger associations with DH in chronic subjects, compared to acute conditions.
While surgical removal of DH is thought to resolve pain syndrome due to unloading of the compressed nerve root, our findings also indicate that resolution of inflammation occurs at 3 months post surgery. Serum levels of MIF, in a small cohort of patients that were analyzed longitudinally from pre- to post-treatment, were found to decrease. A prior study found that MIF expression in herniated discs were higher than in control (cervical trauma) or degenerate by contained disc samples (43), confirming that decreases in MIF levels represents a resolution in cytokine levels in response to treatment. Moderate but significant increases in chemokine levels were also observed in patients post surgery. The increases in the chemokine CCL3/MIP1a was unexpected. Prior studies show that CCL3 expression correlates positively with IVD degeneration and that expression levels are higher in DH tissue compared with contained degenerate discs (44). This finding implies that a decrease in CCL3 levels would be expected when a DH is removed; instead, CCL3 levels increased systemically. Such differences highlight that immune responses are differentially regulated at the tissue and systemic levels. Increases in systemic chemokine subsets may be due to tissue remodeling and wound healing post surgery. The greatest increase observed was for CXCL10 (2.1 fold change, p=0.043), a chemokine with known angiostatic activity (45). Future studies into longer-term changes in chemokine levels will inform whether these elevations resolve with remodeling, or whether such increases are associated with aberrant or impaired neovascularization and surgical responses to treatment. Inefficient healing or prolonged inflammation can drive chronic dysfunction, and therefore studies of inflammation in this patient population may shed important insight into the disease progression and recovery of DH and related back pain.
Some limitations of this study include: 1) The sample size was relatively small compared to the number of serum factors considered, thus increasing the possibility of false negatives. 2) The study did not include measures of disability or other functional indicators to compliment the pain VAS scores. 3) The biochemical analysis was conducted on blood serum samples, which are more dilute than CSF samples and have contributions from all organ systems across the body. 4) The population size in the longitudinal pre- to post- surgery analysis was small, however because it is a repeat measure analysis within the same subjects, it was sufficient to yield significant difference in some factors that exhibited a large effect sizes (Es ~0.9). Replications with a larger sample size when examining the effect of DH interventions is warranted. Nevertheless, this study adds valuable insight into immune mediator changes occurring in DH patients.
In summary, this study is the first to show that DH severity is associated with changes in the circulating levels of inflammatory cytokines in patients and identifies HMGB1, PDGFbb and IL-9 as novel molecular surrogates or mediators of increasing DH severity. We also found that systemic inflammatory profiles have stronger associations with DH in chronic subjects, compared to acute cases. Longitudinal post-surgical analysis yielded resolution in MIF levels, however sustained chemokine upregulation was maintained 3 months post treatment. These findings extend the understanding of how DH severity and subject-specific factors contribute to inflammatory levels in DH patients.
Supplementary Material
Acknowledgments
The authors do not have any conflicts of interest pertaining to this study. The authors thank Chrissy Demers, RN for assistance with subject recruitment. This study was supported in part by NIH R01AR069668, NYS DOH ECRIP Grant, and by institutional support from Columbia University and Northwell Health.
Financial support: This study was supported in part by NIH R01AR069668, NYS DOH ECRIP Grant, and by institutional support from Columbia University and Northwell Health.
Role of the funding source
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, New York State Department of Health or other funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have declared that no conflict of interest exists
References:
- 1.Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012. May 15;37(11):E668–77. [DOI] [PubMed] [Google Scholar]
- 2.Tsarouhas A, Soufla G, Katonis P, Pasku D, Vakis A, Spandidos DA. Transcript levels of major MMPs and ADAMTS-4 in relation to the clinicopathological profile of patients with lumbar disc herniation. Eur Spine J. 2011. May;20(5):781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zigouris A, Batistatou A, Alexiou GA, Pachatouridis D, Mihos E, Drosos D, et al. Correlation of matrix metalloproteinases-1 and -3 with patient age and grade of lumbar disc herniation. J Neurosurg Spine. 2011. February;14(2):268–72. [DOI] [PubMed] [Google Scholar]
- 4.Omarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998. November;78(2):99–105. [DOI] [PubMed] [Google Scholar]
- 5.Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine (Phila Pa 1976). 1984. January-Feb;9(1):7–15. [DOI] [PubMed] [Google Scholar]
- 6.Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine (Phila Pa 1976). 1996. January 15;21(2):235–41. [DOI] [PubMed] [Google Scholar]
- 7.Gronblad M, Virri J, Tolonen J, Seitsalo S, Kaapa E, Kankare J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine (Phila Pa 1976). 1994. December 15;19(24):2744–51. [DOI] [PubMed] [Google Scholar]
- 8.Ma XL, Tian P, Wang T, Ma JX. A study of the relationship between type of lumbar disc herniation, straight leg raising test and peripheral T lymphocytes. Orthop Surg. 2010. February;2(1):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976). 1996. January 15;21(2):218–24. [DOI] [PubMed] [Google Scholar]
- 10.de Goeij M, van Eijk LT, Vanelderen P, Wilder-Smith OH, Vissers KC, van der Hoeven JG, et al. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One. 2013;8(12):e84159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009. October 16;139(2):267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otoshi K, Kikuchi S, Kato K, Sekiguchi M, Konno S. Anti-HMGB1 neutralization antibody improves pain-related behavior induced by application of autologous nucleus pulposus onto nerve roots in rats. Spine (Phila Pa 1976). 2011. May 15;36(11):E692–8. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki N, Sekiguchi M, Shishido H, Kikuchi S, Yabuki S, Konno S. A comparison of pain-related behavior following local application of nucleus pulposus and/or mechanical compression on the dorsal root ganglion. Fukushima J Med Sci. 2011;57(2):46–53. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita M, Ohtori S, Koshi T, Inoue G, Yamauchi K, Suzuki M, et al. Tumor necrosis factor-alpha in the nucleus pulposus mediates radicular pain, but not increase of inflammatory peptide, associated with nerve damage in mice. Spine (Phila Pa 1976). 2008. August 1;33(17):1836–42. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Inoue G, Gemba T, Watanabe T, Ito T, Koshi T, et al. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009. July;18(7):1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002. February;11(1):62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park MS, Lee HM, Hahn SB, Moon SH, Kim YT, Lee CS, et al. The association of the activation-inducible tumor necrosis factor receptor and ligand with lumbar disc herniation. Yonsei Med J. 2007. October 31;48(5):839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Fan W, Liu B, Wang X, Nie L. Th17 lymphocyte levels are higher in patients with ruptured than non-ruptured lumbar discs, and are correlated with pain intensity. Injury. 2013. December;44(12):1805–10. [DOI] [PubMed] [Google Scholar]
- 19.Kraychete DC, Sakata RK, Issy AM, Bacellar O, Santos-Jesus R, Carvalho EM. Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J. 2010;128(5):259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grad S, Bow C, Karppinen J, Luk KD, Cheung KM, Alini M, et al. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cell Mater. 2016. January;31:1–10. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen LM, Schistad E, Jacobsen LM, Roe C, Gjerstad J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav Immun. 2015. May;46:132–6. [DOI] [PubMed] [Google Scholar]
- 22.Moen A, Lind AL, Thulin M, Kamali-Moghaddam M, Roe C, Gjerstad J, et al. Inflammatory Serum Protein Profiling of Patients with Lumbar Radicular Pain One Year after Disc Herniation. Int J Inflam. 2016;2016:3874964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajasekaran S, Tangavel C, K S SVA, Soundararajan DCR, Nayagam SM, Matchado MS, et al. Inflammaging determines health and disease in lumbar discs-evidence from differing proteomic signatures of healthy, aging, and degenerating discs. Spine J. 2020. 01;20(1):48–59. [DOI] [PubMed] [Google Scholar]
- 24.Hashem LE, Roffey DM, Alfasi AM, Papineau GD, Wai DC, Phan P, et al. Exploration of the Inter-Relationships Between Obesity, Physical Inactivity, Inflammation, and Low Back Pain. Spine (Phila Pa 1976). 2018. 09;43(17):1218–24. [DOI] [PubMed] [Google Scholar]
- 25.Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016. January 7;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber KT, Satoh S, Alipui DO, Virojanapa J, Levine M, Sison C, et al. Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol Res. 2015. December;63(1-3):170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001. September;26(17):1873–8. [DOI] [PubMed] [Google Scholar]
- 28.Janelidze S, Ventorp F, Erhardt S, Hansson O, Minthon L, Flax J, et al. Altered chemokine levels in the cerebrospinal fluid and plasma of suicide attempters. Psychoneuroendocrinology. 2013. June;38(6):853–62. [DOI] [PubMed] [Google Scholar]
- 29.Rahyussalim AJ, Zufar MLL, Kurniawati T. Significance of the Association between Disc Degeneration Changes on Imaging and Low Back Pain: A Review Article. Asian Spine J. 2020. April;14(2):245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeVine J, Norvell DC, Ecker E, Fourney DR, Vaccaro A, Wang J, et al. Evaluating the correlation and responsiveness of patient-reported pain with function and quality-of-life outcomes after spine surgery. Spine (Phila Pa 1976). 2011. October 1;36(21 Suppl):S69–74. [DOI] [PubMed] [Google Scholar]
- 31.Fardon DF, Williams AL, Dohring EJ, Murtagh FR, Gabriel Rothman SL, Sze GK. Lumbar disc nomenclature: version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014. November 1;14(11):2525–45. [DOI] [PubMed] [Google Scholar]
- 32.Shah B, Levine M, Alipui DO, Weber KT, Chahine NO. 2017. Orthopedic Reserach Society; San Diego, CA: High Mobility Group Box-1 Promotes Pro-inflammatory Signaling in Human Nucleus Pulposus Cells via Toll-Like Receptor 4. p. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber HE, Hoelscher GL, Bethea S, Ingram J, Cox M, Hanley EN. High-mobility group box-1 gene, a potent proinflammatory mediators, is upregulated in more degenerated human discs in vivo and its receptor upregulated by TNF-α exposure in vitro. Exp Mol Pathol. 2015. June;98(3):427–30. [DOI] [PubMed] [Google Scholar]
- 34.Shah BS, Burt KG, Jacobsen T, Fernandes TD, Alipui DO, Weber KT, et al. High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via toll-like receptor 4-dependent pathway. J Orthop Res. 2019. January;37(1):220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda T, Ozaki M, Kobayashi Y, Kiguchi N, Kishioka S. HMGB1 as a potential therapeutic target for neuropathic pain. J Pharmacol Sci. 2013;123(4):301–5. [DOI] [PubMed] [Google Scholar]
- 36.Presciutti SM, Paglia DN, Karukonda T, Soung dY, Guzzo R, Drissi H, et al. PDGF-BB inhibits intervertebral disc cell apoptosis in vitro. J Orthop Res. 2014. September;32(9):1181–8. [DOI] [PubMed] [Google Scholar]
- 37.Gruber HE, Norton HJ, Hanley EN. Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine (Phila Pa 1976). 2000. September;25(17):2153–7. [DOI] [PubMed] [Google Scholar]
- 38.Pratsinis H, Constantinou V, Pavlakis K, Sapkas G, Kletsas D. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012. June;30(6):958–64. [DOI] [PubMed] [Google Scholar]
- 39.Paglia DN, Singh H, Karukonda T, Drissi H, Moss IL. PDGF-BB Delays Degeneration of the Intervertebral Discs in a Rabbit Preclinical Model. Spine (Phila Pa 1976). 2016. April;41(8):E449–58. [DOI] [PubMed] [Google Scholar]
- 40.Geiss A, Larsson K, Rydevik B, Takahashi I, Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine (Phila Pa 1976). 2007. January 15;32(2):168–73. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhao Y, Li J, Wang S, Liu Y, Nie L, et al. Interleukin-9 Promotes TNF-α and PGE2 Release in Human Degenerated Intervertebral Disc Tissues. Spine (Phila Pa 1976). 2016. November;41(21):1631–40. [DOI] [PubMed] [Google Scholar]
- 42.Polacchini A, Girardi D, Falco A, Zanotta N, Comar M, De Carlo NA, et al. Distinct CCL2, CCL5, CCL11, CCL27, IL-17, IL-6, BDNF serum profiles correlate to different job-stress outcomes. Neurobiol Stress. 2018. February;8:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang H, Yang X, Liu C, Sun Z, Wang X. Effect of NF-kB signaling pathway on the expression of MIF, TNF-alpha, IL-6 in the regulation of intervertebral disc degeneration. J Musculoskelet Neuronal Interact. 2018. December 1;18(4):551–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Biancotto A, Wank A, Perl S, Cook W, Olnes MJ, Dagur PK, et al. Baseline levels and temporal stability of 27 multiplexed serum cytokine concentrations in healthy subjects. PLoS One. 2013;8(12):e76091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011. March 10;317(5):575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.