Extended Data Figure 10. Time courses of de novo TA and precursor production in pseudo-fed-batch cultures of CSY1297 and CSY1298.
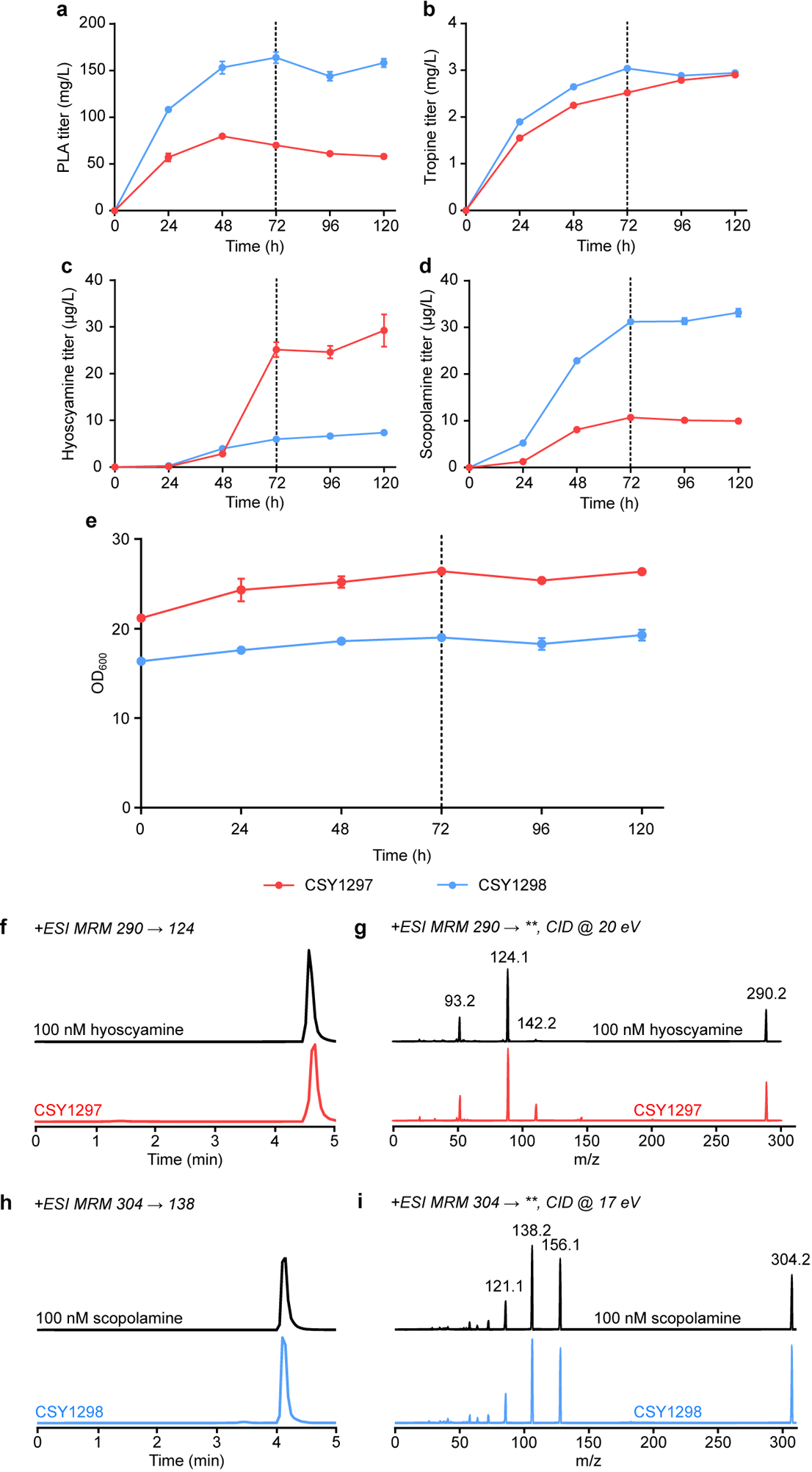
(a-e) TA-producing strains CSY1297 and CSY1298 (with pCS4213) were respectively cultured in non-selective or selective (leucine dropout) media with 50 mM 2-OG and 15 mg/L Fe2+ under pseudo-fed-batch conditions at 25 °C for 120 h. Culture supernatants were sampled for (a) PLA, (b) tropine, (c) hyoscyamine, and (d) scopolamine titers by LC-MS/MS analysis and (e) optical density at 600 nm (OD600) every 24 h. Cultures were supplemented with additional dextrose, glycerol, and amino acids to final concentrations of 2%, 2%, and 1× at 72 h (vertical dotted line). Data indicate the mean of n = 3 biologically independent samples and error bars show standard deviation. Note that no littorine accumulation was observed in any samples. (f-i) LC-MS/MS verification of de novo medicinal TA production in CSY1297 and CSY1298. Panels show LC-MS/MS chromatograms of (f,g) hyoscyamine and (h,i) scopolamine detected in CSY1297 and CSY1298 cultures grown in non-selective media or selective media, respectively (samples from experiment in c and d, 120 h), and of authentic 100 nM chemical standards. Primary MRM transitions used for detection and quantification are shown in f and h; additional characteristic transitions are shown in g and i (‘**’ indicates any detected fragment mass in product ion scan). Traces are representative of n = 3 biologically independent samples.
