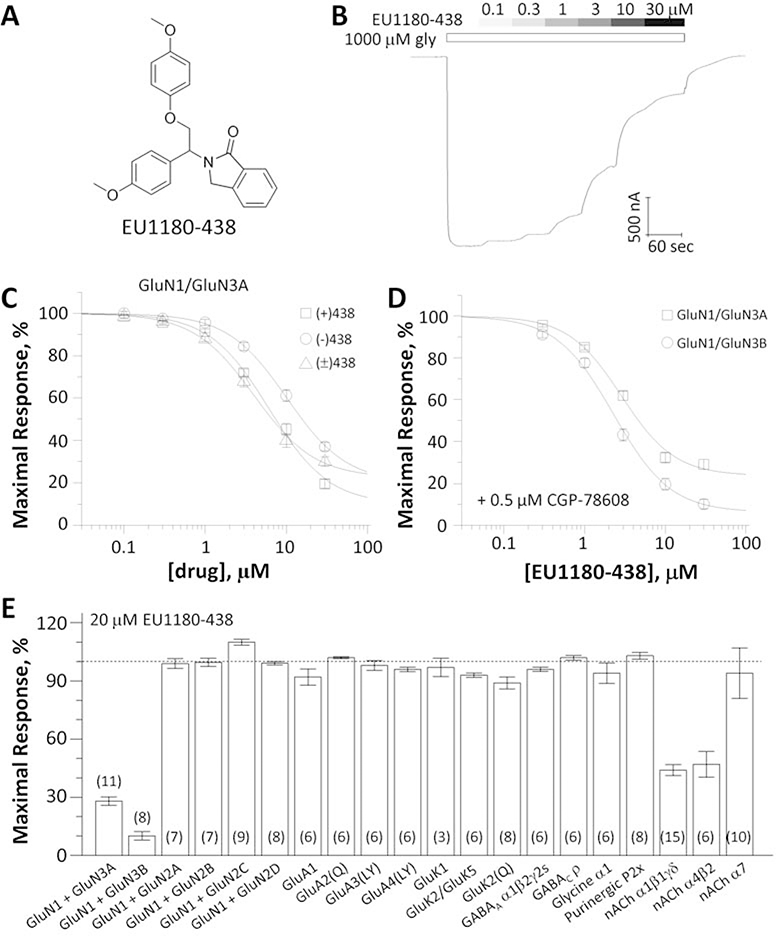
Fig. 1. Evaluation of EU1180–438 as a selective GluN1/GluN3 receptor antagonist.
A, Chemical structure of EU1180–438. B, Representative two-electrode voltage-clamp recording of EU1180–438 inhibition of recombinant GluN1FA,TL/GluN3A receptor responses activated by 1000 μM glycine. C, Concentration-response curves for inhibition of GluN1FA,TL/GluN3A receptor responses activated by 1000 μM glycine by enantiomers and racemate of EU1180–438. (−) EU1180–438 IC50 13 μM, Slope 1.4, minimum 16%; (+) EU1180–438 IC50 7.1 μM, Slope 1.2, minimum 4%; (±) EU1180–438 IC50 4.3 μM, Slope 1.3, minimum 28%). Data are mean ± SEM of 18–26 oocytes. D, Concentration-response relationship for EU1180–438 in 500 nM CGP-78608 plus 100 μM glycine at human GluN1/GluN3A and rat GluN1/GluN3B receptors (IC50 1.8 μM, Slope 1.5, minimum 15% and IC50 2.2 μM, Slope 1.5, minimum 7%). Data are mean ± SEM of 8–9 oocytes. E, Receptor responses to agonists in the presence of 20 μM EU1180–438 normalized to agonist responses in vehicle. GluN1 was co-expressed with GluN2A-D and activated by 100 μM glutamate/100 μM glycine, GluA1–4, GluK1, and GluK2 were activated by 100 μM glutamate, GluK2/K5 was activated by 100 μM AMPA, GABAA and GABAC were activated by 100 μM GABA, Glycine α1 was activated by 100 μM glycine, P2X purinergic receptors were activated by 9 μM ATP, α1β1γδ nicotinic acetylcholine receptors were activated by 1 μM acetylcholine, α4β2 nicotinic acetylcholine receptors were activated by 10 μM acetylcholine, and α7-nicotinic acetylcholine receptors were activated by 300 μM acetylcholine. The numbers of oocytes recorded are in parentheses; Numerical data are given in Supplemental Table 1.