Fig. 5.
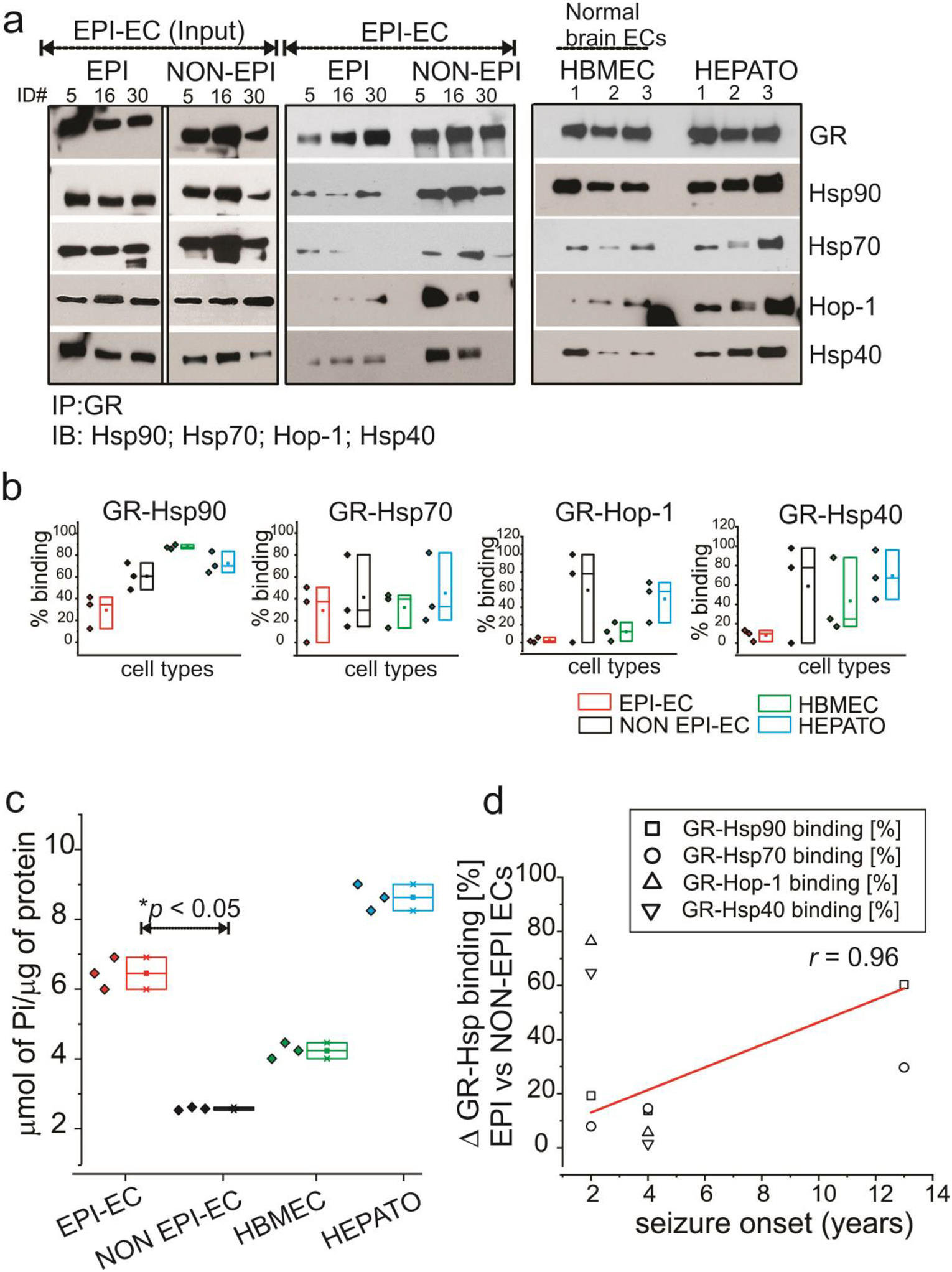
Brain endothelial cell GR-Hsp interactions. (a) The interaction of GR in human brain endothelial cells with the binding partners Hsp90, Hsp70, Hsp40 and Hop is more evident in NON EPI-ECs compared to EPI-ECs (n = 3, #ID, 5, 6, 30). The lanes marked as Input indicate that 1 ⁄5th of total EPI-EC extract loaded. Control ECs (HBMEC, n = 3) and hepatocytes (HEPATO, n = 3) display comparable GR-Hsp binding profiles similar to NON EPI-ECs. The low binding pattern of GR-Hsps in EPI-ECs relative to NON EPI-ECs is evident and suggests faster GR maturation in the EPI-ECs. (b) The quantitation of the GR-Hsp90/Hsp70/Hop-1/Hsp40 interactions depicts a distinct pattern between individual chaperone proteins with GR. (c) The increased maturation of GR is supported by significant increases (*p < 0.05) in ATPase activity of EPI-ECs vs. NON EPI-ECs. The limited ATPase activity in NON EPI-EC is more comparable to HBMEC. (d) Difference in GR-Hsp90, GR-Hsp70, GR-Hop-1 and GR-Hsp40 binding percentages, depicting difference in these interactions (Δ) in EPI and NON-EPI-ECs (n = 3 subjects) were directly correlated (r = 0.96) with individual seizure onset age (years) among the small specimens cohort analyzed. Results are expressed as mean ± SEM by ANOVA, *p < 0.05.
