Figure 1: Mechanisms of regulation of the endocannabinoid signaling system.
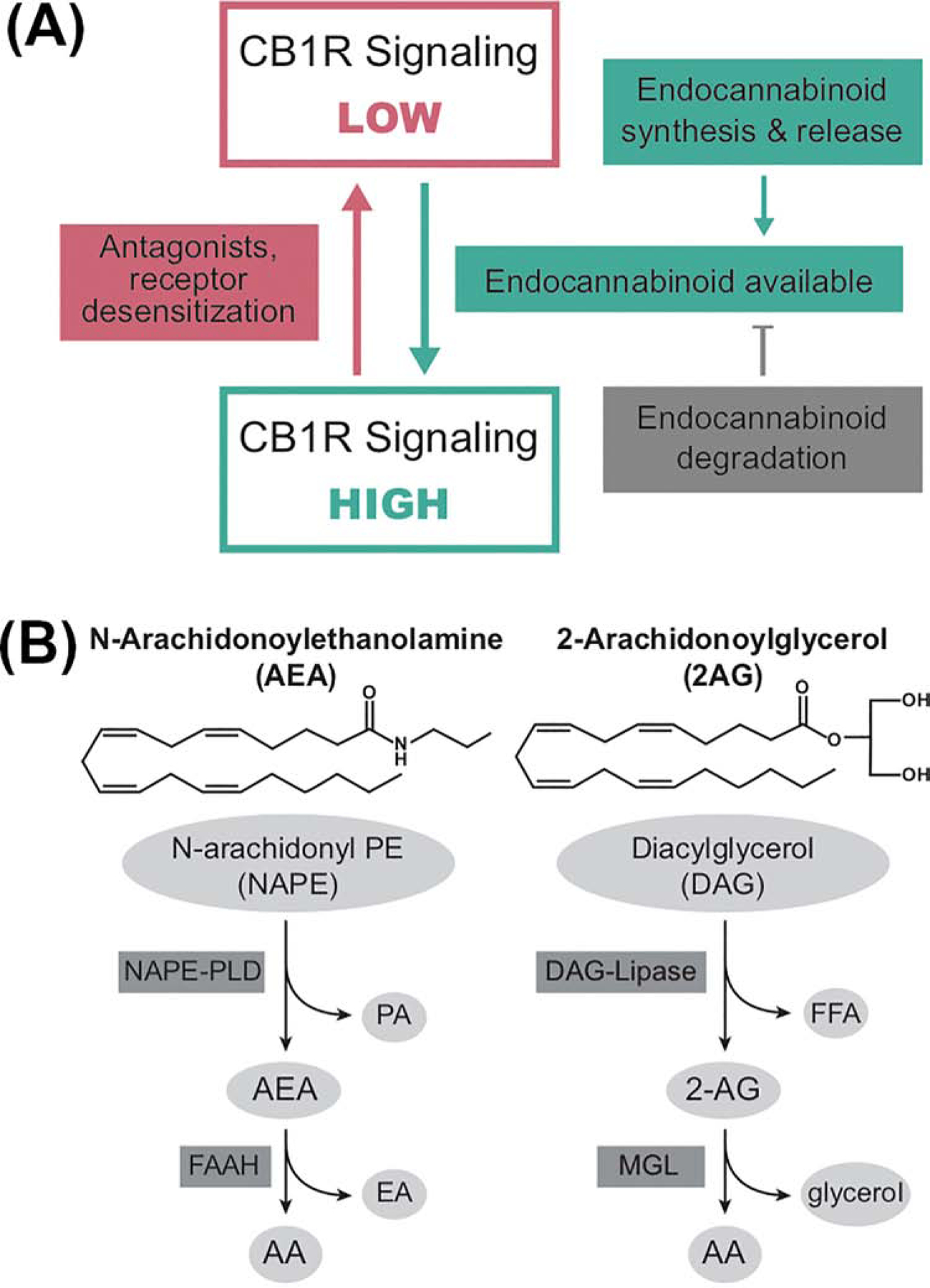
A: The endocannabinoid signaling system (ECSS) is regulated by endocannabinoid and receptor availability. The CB1 subtype of cannabinoid receptor (CB1R) can be in high and low signaling conformations. The primary mechanism regulating the ratio of receptor conformations is endocannabinoid binding to the receptor. Endocannabinoid concentrations are regulated by the relative rates of their synthesis and degradation. Thus, processes that alter the kinetics and expression of these enzymes are also regulators of CB1R activity. In addition, antagonists block the effects of endocannabinoids through occupation of the binding site, while inverse agonists block endocannabinoid binding and promote the low signaling conformation. Persistent CB1R activation, particularly by full agonists, can cause CB1R desensitization temporarily by receptor internalization or post-translational modification or more long-term via receptor down-regulation.
B: Structures and primary pathways for the synthesis and degradation of the endocannabinoids. N-Arachidonoylethanolamine (AEA) is synthesized from a low abundance lipid (N-arachidonoyl-phosphatidylethanolamine; NAPE) through the actions of a NAPE-specific phospholipase D (PLD), with the liberation of phosphatidic acid (PA). AEA is hydrolyzed to free arachidonic acid (AA) and ethanolamine (EA) by the serine hydrolase, fatty acid amide hydrolase (FAAH). 2-Arachidonoylglycerol (2-AG) is synthesized from diacylglycerol (with AA in the sn-2 position) through the actions of diacylglycerol lipase (DAG-Lipase) which results in the release of the fatty acid at the sn-1 position and 2-AG as the products. 2-AG is catabolized by hydrolysis of the ester bond by monoacylglycerol lipase (MGL) with the release of free AA and glycerol.
