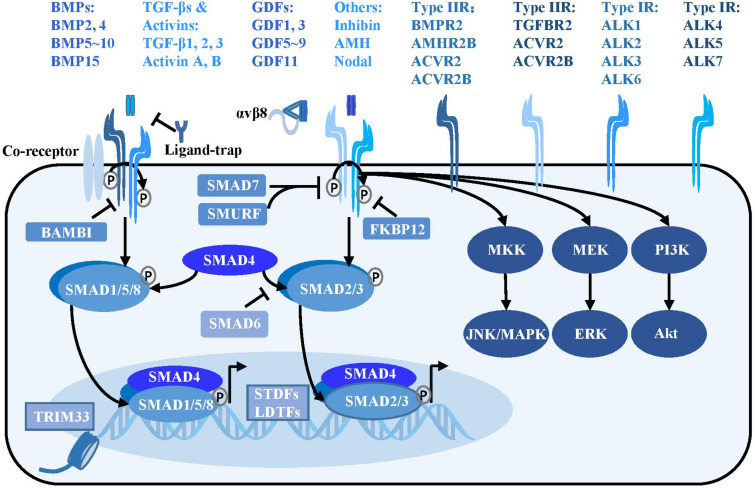
FIGURE 1.
TGF-β signaling pathway. Activated TGF-β ligands with or without αVβ8 integrins bind at serine/threonine protein kinase type II receptors, sometimes with the assistance of the co-receptors such as endoglin. The type II receptors subsequently phosphorylate type I receptors to form a tetrameric receptor complex, which subsequently phosphorylates the SMAD2, SMAD3 or SMAD1, SMAD5, and SMAD8 to form a trimeric complex with SMAD4 in cytoplasm. The SMAD complex then translocates into nucleus and binds at special loci under the guidance of the SDTFs and LDTFs to initiate the transcriptional response. Besides, TRIM33, a modulator of TGF-β signaling, is able to regulate chromatin accessibility and remodeling. In addition to the canonical TGF-β signaling, there are SMAD-independent pathways, such as PI3K/Akt, MEK/ERK and MKK/JNK/MAPK downstream of the TGF-β receptors. TGF-β signaling is negatively regulated at multiple levels. Various ligand traps (noggin, chordin, follistatin, gremlin, coco, and cerberus) can prevent TGF-β ligands from binding to receptors, while FKBP12 and BAMBI can dock at cytoplasmic domain of TGF-β type I receptors to inhibit TGF-β signaling. In addition, inhibitory SMADs including SMAD6 and SMAD7 play a critical function in suppressing the SMAD-mediated signaling.