Sir,
The COVID-19 pandemic has stalled the world and catapulted the global health systems into unprecedented chaos. More than 200 countries have been affected by this pandemic, resulting in 2.54 million cases in a short period of time and >0.17 million deaths (as of April 23, 2020), with a mere 0.7 million recoveries1. The movement of COVID-19 hotspot from China to Europe, and now to the USA, has been partly due to the staggered restrictions in global travel and partly due to potent transmission through asymptomatic carriers2.
India, with 21,393 cases and 681 deaths (as of April 23, 2020)1, had the lowest figures for any country of the comparable population (0.5 deaths per million population). International travellers or their close contacts formed the majority of initially reported cases. The delayed onset of COVID-19 in India has given it an edge, which allowed it to impose severe restrictions to contain the local spread 3,4,5.
In our in-depth analyses of 1500+ genomes, variability among clinical isolates was shown along the timeline, leading to distinct clustering of SARS-CoV-2 across the globe (unpublished observation). It was predicted, based on the aggregation propensity of the spike protein in the Wuhan and other isolates of SARS-CoV-2, that this virus would exhibit very high transmissibility and confer survival fitness6,7. Genetic diversity of the virus increases with disease progression and can be utilized to model the evolution and propagation of the disease6 Recently, phylogenetic network analysis of 160 SARS-CoV-2 genome samples showed a parallel evolution of the virus and its evolutionary selection in their human hosts8. Similar whole-genome analyses of the Indian isolates and their comparison with global isolates can provide a better understanding of dominant clades within the population and unveil targets for developing specific interventions.
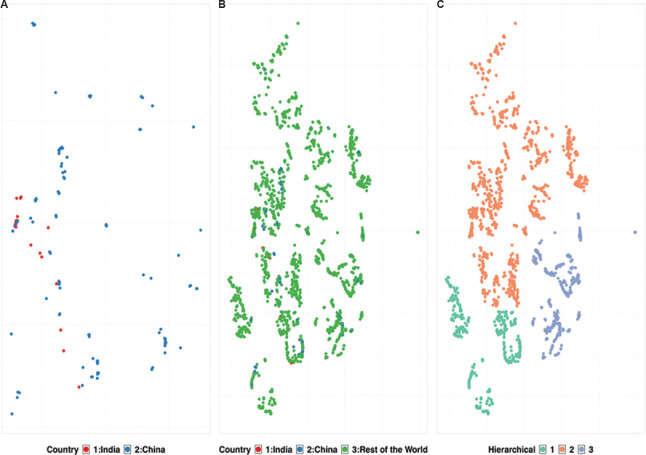
In the present study, machine learning-based t-SNE analysis of global clinical isolates has been utilized to segregate the clinical isolates into clusters while accommodating the outliers9,10. Whole-genome analysis of 3968 global isolates obtained from GISAID (Global initiative on sharing all influenza data)11, including 25 SARS-CoV-2 genomes sequenced in India [next-genome sequencing (NGS) data submitted by the ICMR-National Institute of Virology, Pune, India] and presented in (Figure 1) (Supplementary Fig. 1 (1.3MB, tif) (available from http://www.ijmr.org.in/articles/2020/151/5/images/IndianJMedRes_2020_151_5_474_284485_sm5.pdf) and [Supplementary Table 1 (available from http://www.ijmr.org.in/articles/2020/151/5/images/IndianJMedRes_2020_151_5_474_284485_sm6.pdf)), was an attempt to dissect the global genome diversity and also critically evaluate the placement of Indian isolates to understand the COVID-19 pandemic in India.
Fig. 1.
Whole-genome-based t-SNE clustering of 3968 clinical isolates. (A) Comparative genome-based clustering of Indian isolates (red) with Chinease isolates (blue). (B) Comapartive genome-based clustering of Indian isolates (red) and Chinese isolates (blue) with rest of the world (green). (C) Diversity in clinical isolates showing three distinct clustering using hierarchical clustering on the t-SNE clusters. (t-SNE: https://github.com/jdonaldson/rtsne).
Supplementary Table I.
Details of Indian samples along with their origin and placement in Hierarchical clusters. Genomic sequences were retrieved from GISAID (https://www.gisaid.org)
| Accession ID | Contact History | Gender | Age | Virus name | Location | Collection date | Hierarchical Cluster ID |
|---|---|---|---|---|---|---|---|
| EPI_ISL_413523 | Travel history to China | Male | 23 | hCoV-19/India/1-31/2020 | Asia/India/Kerala | 2020-1-31 | 2 |
| EPI_ISL_420543 | Italian tourist | Female | 73 | hCoV-19/India/763/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420544 | Vero CCL81 isolate P1 | NA | NA | hCoV-19/India/2020763/2020 | Asia/India | 2020 | 1 |
| EPI_ISL_420545 | Italian tourist | Female | 77 | hCoV-19/India/770/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420546 | Vero CCL81 isolate P1 | NA | NA | hCoV-19/India/2020770/2020 | Asia/India | 2020 | 1 |
| EPI_ISL_420547 | Italian tourist | Female | 70 | hCoV-19/India/772/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420548 | Vero CCL81 isolate P1 | NA | NA | hCoV-19/India/2020772/2020 | Asia/India | 2020 | 2 |
| EPI_ISL_420549 | Italian tourist | Female | 65 | hCoV-19/India/773/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420550 | Vero CCL81 isolate P1 | NA | NA | hCoV-19/India/2020773/2020 | Asia/India | 2020 | 2 |
| EPI_ISL_420551 | Indian contact of Italian tourist | Male | 59 | hCoV-19/India/777/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420552 | Vero CCL81 isolate P1 | NA | NA | hCoV-19/India/2020777/2020 | Asia/India | 2020 | 2 |
| EPI_ISL_420553 | Italian tourist | Male | 66 | hCoV-19/India/781/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420554 | Vero CCL81 isolate P1 | NA | NA | hCoV-19/India/2020781/2020 | Asia/India | 2020 | 2 |
| EPI_ISL_420555 | Indian contact of Indian Patient having travel history to Italy | Female | 37 | hCoV-19/India/c32/2020 | Asia/India | 2020-3-3 | 2 |
| EPI_ISL_420556 | Vero CCL81 isolate P1 | NA | hCoV-19/India/2020c32/2020 | Asia/India | 2020 | 1 | |
| EPI_ISL_421662 | Indian citizen sampled at Iran | Male | 68 | hCoV-19/India/1073/2020 | Asia/India | 2020-3-10 | 2 |
| EPI_ISL_421663 | Indian citizen sampled at Iran | Male | 45 | hCoV-19/India/1093/2020 | Asia/India | 2020-3-10 | 2 |
| EPI_ISL_421664 | Indian citizen sampled at Iran | Male | 72 | hCoV-19/India/1100/2020 | Asia/India | 2020-3-10 | 2 |
| EPI_ISL_421665 | Indian citizen sampled at Iran | Male | 43 | hCoV-19/India/1104/2020 | Asia/India | 2020-3-10 | 1 |
| EPI_ISL_421666 | Indian citizen sampled at Iran | Female | 54 | hCoV-19/India/1111/2020 | Asia/India | 2020-3-10 | 2 |
| EPI_ISL_421667 | Indian citizen sampled at Iran | Male | 66 | hCoV-19/India/1115/2020 | Asia/India | 2020-3-10 | 2 |
| EPI_ISL_421669 | Indian citizen sampled at Iran | Female | 70 | hCoV-19/India/1616/2020 | Asia/India | 2020-3-12 | 2 |
| EPI_ISL_421670 | Indian citizen sampled at Iran | Female | 50 | hCoV-19/India/1617/2020 | Asia/India | 2020-3-12 | 2 |
| EPI_ISL_421671 | Indian citizen sampled at Iran | Female | 55 | hCoV-19/India/1621/2020 | Asia/India | 2020-3-12 | 2 |
| EPI_ISL_421672 | Indian citizen sampled at Iran | Male | 59 | hCoV-19/India/1644/2020 | Asia/India | 2020-3-12 | 2 |
The initial cases reported from India had a travel history to China, which explained its position in a Chinese cluster5 (Fig. 2). The travel ban from China to India, in early February 2020, has prevented the large-scale spill-over directly from China to the Indian Sub-continent. However, various isolates transmitted from other South-East Asian countries might fall in the same cluster. The overlap of Indian samples majorly with European samples (Supplementary Fig. 1 (1.3MB, tif) , Panel III) reiterated the fact that the delayed travel restriction from the European hotspot regions affected not just India but also many countries.
Fig. 2.
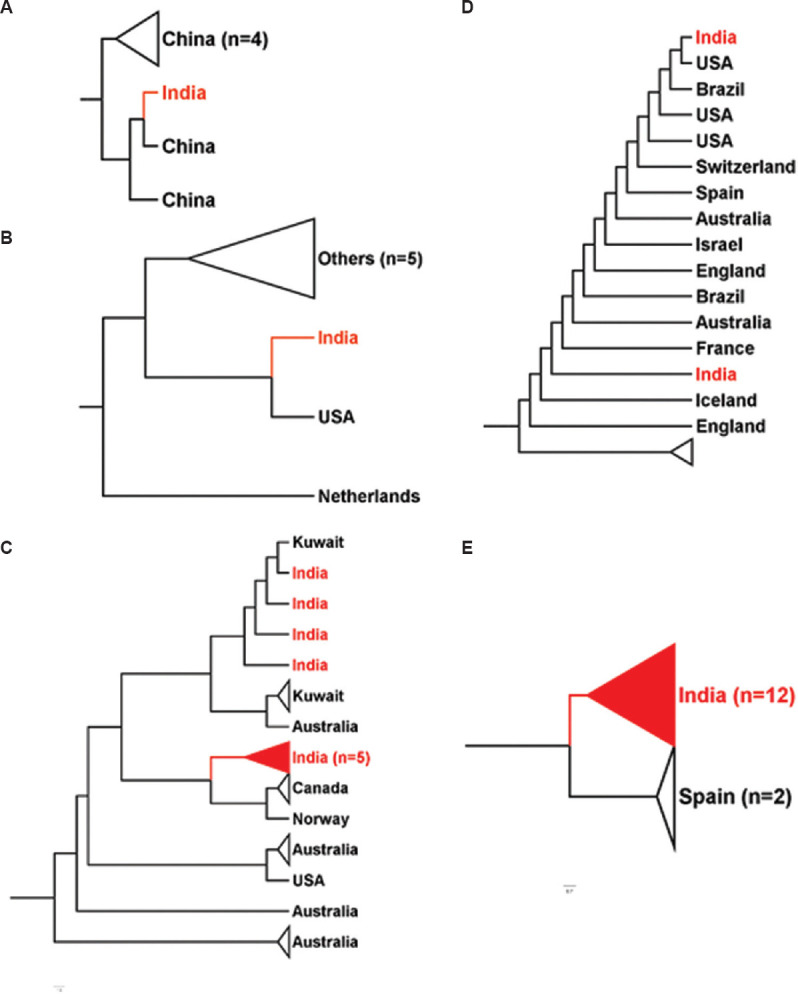
Position of various Indian isolates with other nations. (A-E) Clustering of SARS-CoV-2 genome sequences from India (red) with other nations around the globe. Indian samples clustered with samples from different nations – China, Kuwait, Canada, USA and Spain in whole-genome-based clustering. Figures were generated using FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
Hierarchical-based clustering further yielded exciting outcomes on the inter-continent transmission of COVID-19. The segregation of SARS-CoV-2 genomes into three clades indicates the emergence of evolutionary diversity (Fig. 1C). The heterogeneity of these clusters, grouped along with Chinese counterparts, validates a global spill-over event originating from Wuhan5,12. Hierarchical cluster 2 in Supplementary Figure 1 (1.3MB, tif) Panel II (coloured by the continents) indicates the introduction of SARS-CoV-2 in India from the European, other Asian and North American nations (Supplementary Fig. 1 (1.3MB, tif) ). Detailed comparative analysis of Indian isolates with respect to other countries showed its close relationship with samples from China, USA, Canada, Spain and Kuwait, suggestive of exposure to COVID-19 due to travel history from these nations (Fig. 2). However, limited genome sequences from India make it difficult to differentiate and ascertain global transmission and transmission within the country.
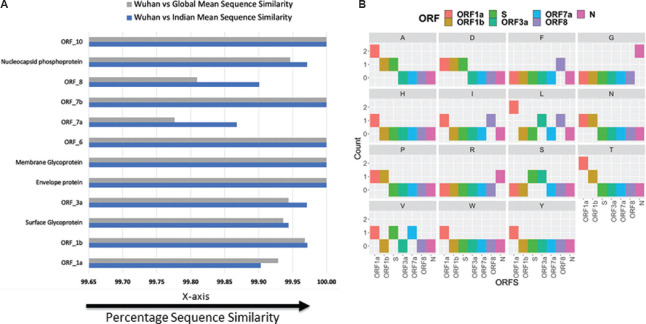
The conservation of an amino acid in any protein sequence denotes its functional importance13,14 as it undergoes fewer amino acid replacements or is more likely to substitute amino acids with similar biochemical properties. The amino acid conservation is inversely proportionate to the evolutionary rate. This is a valuable gauge of the evolutionary divergence and the analogous genomic regions. Sequence similarity between the open reading frames (ORFs) of Indian isolates and the initial sample collected in Wuhan unravels conservation in five ORFs corresponding to envelope protein, membrane glycoprotein, ORF6, ORF7b and ORF10 proteins (Fig. 3A). On the contrary, a number of mutations were observed in ORF1a, ORF1b, spike protein (surface glycoprotein), ORF3a, ORF7a, ORF8 and nucleocapsid phosphoprotein (Supplementary Fig. 2 (1.8MB, tif) (available from http://www.ijmr.org.in/articles/2020/151/5/images/IndianJMedRes_2020_151_5_474_284485_sm7.pdf and Supplementary Table II (available from http://www.ijmr.org.in/articles/2020/151/5/images/IndianJMedRes_2020_151_5_474_284485_sm8.pdf)). Mean similarity calculated for these ORFs revealed that ORF1a in the Indian isolates was less conserved (more mutated) compared to global isolates (Fig. 3A and Supplementary Table III (available from http://www.ijmr.org.in/articles/2020/151/5/images/IndianJMedRes_2020_151_5_474_284485_sm9.pdf)). In all other ORFs, a relatively higher conservation was observed among Indian isolates compared to Wuhan strain. When compared with global isolates, Indian isolates have higher entropy for changes in ORF 1a and ORF 1b (Supplementary Fig. 3 (1.3MB, tif) (available from http://www.ijmr.org.in/articles/2020/151/5/images/IndianJMedRes_2020_151_5_474_284485_sm10.pdf)).. Further, qualitative analysis of mutations in non-conserved ORFs showed that each type of amino acid had undergone mutation in the Indian isolates (Fig. 3B). These mutations could be a major contributing factor for the separation of Indian isolates into three distinct clusters. Higher sampling rate driven by NGS of the Indian isolates would help in better understanding of actual variability in SARS-CoV-2 and assist both in identifying better diagnostic markers and in developing specific interventions in terms of vaccine candidates and drug targets.
Fig. 3.
Sequence similarity and mutation analysis of open reading frames. (A) Comparison of mean sequence similarity for open reading frames between Indian and global isolates with Wuhan strain. (B) Qualitative analysis on type of mutations occurring in non-conserved open reading frames (ORFs) of Indian isolates compared to Wuhan strain.
Supplementary Table II.
Specific high frequency (>=10%) mutations in individual ORFs in Indian isolates compared with reference strain (Wuhan_IPBCAMS-WH-01_2019_EPI_ISL_402123). The sequences with less than 25% gaps were selected for all the studies. Genomic sequences were retrieved from GISAID (https://www.gisaid.org)
| ORF | Position | Amino acid in reference | Mutated amino acid | Mutation |
|---|---|---|---|---|
| ORF1a | 207 | Arginine (R) | Cysteine | R->C |
| ORF1a | 378 | Valine (V) | Isoleucine (I) | V->I |
| ORF1a | 1515 | Serine (S) | Phenylalanine (F) | S->F |
| ORF1a | 2796 | Methionine (M) | Isoleucine (I) | M->I |
| ORF1a | 3606 | Leucine (L) | Phenylalanine (F) | L->F |
| ORF1b | 314 | Proline (P) | Leucine (L) | P->L |
| S | 614 | Aspartic acid (D) | Glycine (G) | D->G |
| ORF7a | 74 | Valine (V) | Phenylalanine (F) | V->F |
Supplementary Table III.
Comparison of mean sequence similarity for ORFs between Indian and global isolates with Wuhan strain
| Column 1 ORF |
Column 2 Name |
Wuhan vs Indian | Wuhan vs Global | ||
|---|---|---|---|---|---|
| Indian Mean Similarity | Indian GMean Similarity | Global Mean Similarity | Global GMean Similarity | ||
| ORF5 | ORF_1a | 99.90 | 99.90 | 99.93 | 99.93 |
| ORF1 | ORF_1b | 99.97 | 99.97 | 99.97 | 99.97 |
| ORF2 | Surface Glycoprotein | 99.94 | 99.94 | 99.94 | 99.94 |
| ORF3 | ORF_3a | 99.97 | 99.97 | 99.94 | 99.94 |
| ORF4 | Envelope protein | 100.00 | 100.00 | 100.00 | 100.00 |
| ORF6 | Membrane Glycoprotein | 100.00 | 100.00 | 100.00 | 100.00 |
| ORF7 | ORF_6 | 100.00 | 100.00 | 100.00 | 100.00 |
| ORF8 | ORF_7a | 99.87 | 99.87 | 99.78 | 99.78 |
| ORF9 | ORF_7b | 100.00 | 100.00 | 100.00 | 100.00 |
| ORF10 | ORF_8 | 99.90 | 99.90 | 99.81 | 99.81 |
| ORF11 | Nucleocapsid phosphoprotein | 99.97 | 99.97 | 99.95 | 99.95 |
| ORF12 | ORF_10 | 100.00 | 100.00 | 100.00 | 100.00 |
Evolutionary divergence, corroborated by epidemiological data, is a valuable tool to implement appropriate measures against this pandemic. The population density of India and the presence of functionally distinct isolates in the Indian population raise concerns and warrant an urgent need for higher sampling rate for better assessment of the evolution of SARS-CoV-2 in India. The situation is further confounded by the fact that many of these Indian isolates submitted in databanks include those of Indians living in Iran, Italian tourists visiting India, and also contains samples cultured in vitro.
In conclusion, a whole-genome diversity analysis of 3968 global clinical isolates, including 25 isolates sequenced in India, of SARS-CoV-2 was done. The variations in different open reading frames (ORFs) of SARS-CoV-2, which drives the formation of distinct Indian clusters and functional heterogeneity, were highlighted. Five ORFs corresponding to envelope protein, membrane glycoprotein, ORF6, ORF7b and ORF10 were found to be highly conserved, while a number of mutations were observed in ORF1a, ORF1b, spike protein, ORF3a, ORF7a, ORF8 and nucleocapsid phosphoprotein. Generating diverse genomic datasets will provide insight into the propagation dynamics of COVID-19, leading to a better understanding of pathogenesis and evolution of SARS-CoV-2, which will eventually lead to better intervention methods.
SUPPLEMENTARY INFORMATION
Mapping the genomic landscape and diversity of COVID-19 based on >3950 clinical isolates of SARS-CoV-2: Likely origin and transmission dynamics of isolates sequenced in India
Position of Indian genome sequences in sub-cluster a, b, and c with respect to other global genome sequences. Panel I shows presence of Indian isolates in three distinct sub-clusters with respect to Chinese isolates and remaining global isolates. Panel II highlights the placement of Indian clusters in two of the three hierarchical clusters (a, b, and c) obtained from t-SNE whole genome clustering of 3968 sequences. Panel III displays the prevalence of samples from various continents. Continent codes; Africa (Green), Australia (Orange), North America (Blue), Asia (Light purple), Europe (Yellow) and South America (Pink).
Comparison of all Indian SARS-CoV-2 genomes with Wuhan strain (first collected sample) shows variation in ORF1a and 1b protein, surface glycoprotein, ORF3a protein, ORF7a protein, ORF8 and nucleocapsid protein.
Differential entropy plots for Indian vs global isolates. Mutational entropy of each amino acid position in all ORFs calculated for Indian and global isolates with respect to reference strain (Wuhan_IPBCAMS-WH-01_2019_EPI_ISL_402123). In global isolates, Indian samples have not been included to highlight differential entropy. Genomic sequences were retrieved from GISAID (https://www.gisaid.org).
Acknowledgment
The seventh author (SEH) acknowledges Department of Biotechnology, Government of India for funding support (BT/PR23099/NER/95/632/2017), (BT/PR23155/NER/95/634/2017). SEH is a JC Bose National Fellow, Department of Science and Technology, Government of India & Robert Koch Fellow, Robert Koch Institute, Berlin. The first author (HS) is a recipient of Women Scientist fellowship, Department of Health Research and the second (JS) & fourth (SJ) authors received Young Scientist fellowships from the Department of Health Research, Ministry of Health and Family Welfare, Government of India. The sixth author (JAS) received UGC Startup grant and the third author (MK) received Silver Jubilee Post-Doctoral fellowship from Jamia Hamdard, New Delhi. Authors acknowledge the Originating and Submitting Laboratories for their sequences and meta-data shared through GISAID on which this study is based. Authors acknowledge BioInception Pvt. Ltd, for providing their proprietary data analysis pipeline and platform.
Footnotes
Supplementary material available from http://www.ijmr.org.in/article.asp?issn=0971-5916;year=2020;volume=151;issue=5;spage=474;epage=478;aulast=Singh
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation report-94. Geneva: WHO; 2020. [Google Scholar]
- 2.Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King county, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–81. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal S, Bhatnagar T, Arinaminpathy N, Agarwal A, Chowdhury A, Murhekar M, et al. Prudent public health intervention strategies to control the coronavirus disease 2019 transmission in India: A mathematical model-based approach. Indian J Med Res. 2020;151:190–9. doi: 10.4103/ijmr.IJMR_504_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta N, Praharaj I, Bhatnagar T, Thangaraj JWV, Giri S, Chauhan H, et al. Severe acute respiratory illness surveillance for coronavirus disease 2019, India, 2020. Indian J Med Res. 2020;151:236–40. doi: 10.4103/ijmr.IJMR_1035_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadav PD, Potdar VA, Choudhary ML, Nyayanit DA, Agrawal M, Jadhav SM, et al. Full-genome sequences of the first two SARS-CoV-2 viruses from India. Indian J Med Res. 2020;151:200–9. doi: 10.4103/ijmr.IJMR_663_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamal S, Singh J, Sheikh JA, Singh H, Khubaib M, Kohli S, et al. Molecular Analyses of Over Hundred Sixty Clinical Isolates of SARS-CoV-2: Insights on Likely Origin, Evolution and Spread, and Possible Intervention Preprints. 2020 doi: 1020944/preprints2020030320v1. [Google Scholar]
- 7.Sheikh JA, Singh J, Singh H, Jamal S, Khubaib M, Kohli S, et al. Emerging genetic diversity among clinical isolates of SARS-CoV-2: Lessons for today? Infect Genet Evol. 2020;84:104330. doi: 10.1016/j.meegid.2020.104330. doi:10.1016/j.meegid.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117:9241–3. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Maaten. Accelerating t-SNE using tree-based algorithms. J Mach Learn Res. 2014;15:3221–45. [Google Scholar]
- 11.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–2. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdar WS, Thornton JM. Protein-protein interfaces: Analysis of amino acid conservation in homodimers. Proteins. 2001;42:108–24. [PubMed] [Google Scholar]
- 14.Wu TT, Kabat EA. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970;132:211–50. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Position of Indian genome sequences in sub-cluster a, b, and c with respect to other global genome sequences. Panel I shows presence of Indian isolates in three distinct sub-clusters with respect to Chinese isolates and remaining global isolates. Panel II highlights the placement of Indian clusters in two of the three hierarchical clusters (a, b, and c) obtained from t-SNE whole genome clustering of 3968 sequences. Panel III displays the prevalence of samples from various continents. Continent codes; Africa (Green), Australia (Orange), North America (Blue), Asia (Light purple), Europe (Yellow) and South America (Pink).
Comparison of all Indian SARS-CoV-2 genomes with Wuhan strain (first collected sample) shows variation in ORF1a and 1b protein, surface glycoprotein, ORF3a protein, ORF7a protein, ORF8 and nucleocapsid protein.
Differential entropy plots for Indian vs global isolates. Mutational entropy of each amino acid position in all ORFs calculated for Indian and global isolates with respect to reference strain (Wuhan_IPBCAMS-WH-01_2019_EPI_ISL_402123). In global isolates, Indian samples have not been included to highlight differential entropy. Genomic sequences were retrieved from GISAID (https://www.gisaid.org).