Abstract
Objective: Whether or not emergent decompression/fusion surgery for paralysis caused by metastatic spinal tumors of unknown origin improves patient neurological outcome and survival remains unclear. This study aimed to evaluate the clinical outcomes of emergent decompression/fusion surgery for paralysis caused by spinal tumors of unknown or not previously diagnosed origin.
Patients and Methods: Data from the medical records of 11 patients with spinal tumors of unknown origin (study group) were compared with those of 15 patients with metastatic spinal tumors of known origin (control group). The outcome measures were postoperative performance status, motor function evaluated with the Frankel grade, and actual survival after surgery as compared with the estimated survival calculated using the Tokuhashi score. χ2 analyses were performed to evaluate differences between the groups.
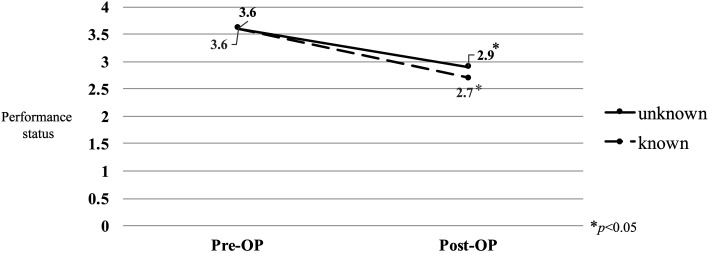
Results: The mean performance status was 3.6 preoperatively, which improved to 2.9 postoperatively (P<0.05), in the unknown origin group and 3.6 preoperatively, which improved to 2.7 postoperatively (P<0.05), in the control group. Seven patients (64%) in the unknown origin group showed improvement in paralysis by ≥1 Frankel grade. By contrast, only 4 patients (27%) in the control group showed improvement in paralysis. The unknown origin group tended to show better improvement (P=0.05). All the patients in the unknown origin group underwent adjuvant therapy after definitive diagnosis following surgery. The unknown origin group showed a slight tendency toward better survival than toward the estimated survival.
Conclusion: Emergent decompression/fusion surgery for patients with paralysis caused by metastatic tumors of unknown origin is potentially useful for diagnosing tumor origin and improving neurological outcomes and performance status, and thus for extending survival.
Keywords: metastatic spinal tumor, emergent surgery, neurological outcome
Introduction
Metastatic spinal tumors occur in approximately 20% of patients with cancer1). Recent developments in multidisciplinary oncology, including novel chemotherapeutic agents, molecular targeted drugs, biologics, and heavy-particle beam therapy, have improved cancer patient survival, resulting in a marked increase in the incidence of metastatic spinal tumors. Spinal metastasis is a serious problem that significantly affects the quality of life of patients with cancer2).
The general principle involved in the treatment of metastatic spinal tumors is a multidisciplinary approach that includes decompression/fusion surgery based on patient symptoms. We regularly have opportunities to perform emergency surgery for patients with urgent paralysis caused by metastatic spinal tumor of unknown origin. Few reports of such cases have been published. Whether emergent decompression/fusion surgery for paralysis caused by metastatic spinal tumors of unknown origin improves patient survival remains unclear. We hypothesized that better clinical outcomes can be obtained with emergent decompression/fusion surgery for paralysis caused by metastatic spinal tumors of unknown origin than for that caused by metastatic spinal tumors of known origin. This is because patients with metastatic spinal tumors of unknown origin could get the opportunity to undergo primary multidisciplinary treatment after emergent surgery. This study aimed to evaluate the clinical outcomes of emergent decompression/fusion surgery for paralysis caused by metastatic spinal tumors of unknown or not previously diagnosed origin.
Patients and Methods
Study design
This was a retrospective case-control study.
Demographics
The present series included 11 consecutive patients (8 men and 3 women) who underwent emergent decompression/fusion surgery for acute paralysis caused by spinal tumors of unknown origin. The mean age at treatment initiation in our hospital was 65.1 years (range, 46–81 years; Table 1). Fifteen patients with acute paralysis caused by metastatic spinal tumors of known origin (7 men and 8 women) served as the control group. The mean age at treatment initiation in our hospital was 54.6 years (range, 26–82 years; Table 2). The known primary tumors included lung cancer (n=4), thyroid cancer (n=3), breast cancer (n=2), cervical cancer (n=1), testicular cancer (n=1), renal cell carcinoma (n=1), malignant melanoma (n=1), adenoid cystic carcinoma (n=1), and angiosarcoma of the breast (n=1; Table 2). Informed consent was obtained from all patients before their participation in the study.
Table 1. Characteristics of patients with tumors of unknown origin.
| No. | Age | Sex | Level of paralysis | Initialsymptom | Durationfrom initial symptomto paralysis(months) | Tokuhashi’sscore | Surgicalstrategy | Operativetime(min) | Estimatedblood loss (ML) | Perioperative complications | Pathological diagnosis | Survivalperiod(months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | T6 | Para paralysis | 0 | 4 | PD | 155 | 200 | - | Malignant lymphoma | 12 |
| 2 | 65 | M | T2 | Back pain | 0.5 | 5 | PD | 372 | 1,000 | - | Malignant lymphoma | 9 |
| 3 | 49 | M | T3 | Para paralysis | 0 | 6 | PD | 280 | 1,500 | - | leukemia | 3 |
| 4 | 66 | M | T2 | Back pain | 4 | 3 | 178 | 360 | - | Renal pelvic cancer | 6 | |
| 5 | 49 | M | T1 | Back pain | 1.5 | 9 | 309 | 290 | Pulmonary embolism | Prostate cancer | 29 | |
| 6 | 81 | F | T4 | Back pain | 0.5 | 3 | 200 | 165 | Pneumonia | Malignant lymphoma | 1 | |
| 7 | 62 | F | T7 | Back pain | 2 | 4 | 266 | 450 | - | Lung cancer | 20 | |
| 8 | 80 | M | T10 | Back pain | 5 | 2 | 284 | 910 | - | Lung cancer | 20 | |
| 9 | 72 | M | C6 | Muscle weakness of the arm | 0 | 9 | 605 | 500 | Pneumonia | Liver cyst adenocarcinoma | 19 | |
| 10 | 71 | F | L1 | Gait disturbance | 0 | 10 | PD | 98 | 120 | - | Malignant lymphoma | 32 |
| 11 | 46 | M | T7 | Back pain | 2 | 9 | PD | 247 | 995 | - | Synovial sarcoma | 4 |
| Mean | 65.1 | 1.9 | 5.8 | 272 | 590 | 14 | ||||||
PD: posterior decompression; PDF: posterior decompression with instrumented fusion.
Table 2. Characteristics of patients with tumors of known origin.
| No. | Age | Sex | Level of paralysis | Initialsymptom | Durationfrom initial symptomto paralysis(months) | Tokuhashi’sscore | Surgicalstrategy | Operativetime(min) | Estimatedblood loss (ML) | Perioperative complications | Primarydiagnosis | Survivalperiod(months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | T9 | Back pain | 0 | 9 | PF | 201 | 235 | - | Thyroid cancer | 14 |
| 2 | 57 | F | T10 | Back pain | 5 | 1 | 175 | 200 | - | Lung cancer | 9 | |
| 3 | 48 | M | T3 | Numbness of the leg | 0 | 4 | 206 | 130 | - | Lung cancer | 8 | |
| 4 | 26 | M | T9 | Back pain | 0.5 | 7 | PD | 147 | 100 | - | Testis cancer | 30 |
| 5 | 65 | F | T1 | Para paralysis | 0 | 8 | PF | 355 | 430 | - | Thyroid cancer | 9 |
| 6 | 74 | F | T4 | Back pain | 1 | 7 | 237 | 350 | - | Breast cancer | 13 | |
| 7 | 57 | M | T5 | Back pain | 1.5 | 8 | 257 | 190 | - | Thyroid cancer | 7 | |
| 8 | 66 | M | T7 | Gaitdisturbance | 0 | 5 | PF | 264 | 600 | - | Lung cancer | 6 |
| 9 | 62 | F | T10 | Back pain | 0.5 | 7 | 339 | 384 | - | Cervical cancer | 5 | |
| 10 | 71 | F | T7 | Gaitdisturbance | 0 | 6 | 227 | 92 | - | Malignant melanoma | 2 | |
| 11 | 42 | M | C3 | Neck pan | 1 | 7 | 428 | 345 | Urinary tract infection | Adenoid cystic carcinoma | 5 | |
| 12 | 39 | F | T3 | Back pain | 2 | 4 | 379 | 4,100 | - | Angiosarcoma of the breast | 2 | |
| 13 | 82 | F | T5 | Back pain | 1 | 8 | PD | 195 | 690 | - | Breast cancer | 15 |
| 14 | 64 | M | T3 | Back pain | 7 | 3 | 232 | 690 | Surgical site infection | Lung cancer | 12 | |
| 15 | 15 | M | T2 | Back pain | 3 | 7 | 424 | 583 | - | Renal cell carcinoma | 4 | |
| Mean | 55 | 2.3 | 6.1 | 271 | 608 | 9.4 | ||||||
PD: posterior decompression; PDF: posterior decompression with instrumented fusion; PF: posterior fusion.
Data from the patients’ clinical records were used to evaluate the level of paralysis, initial symptoms, duration from initial symptom to paralysis, and preoperative severity of paralysis and to compare estimated blood loss and operative time between the groups. Perioperative complication data were also collected from the patients’ clinical records.
Outcome measures
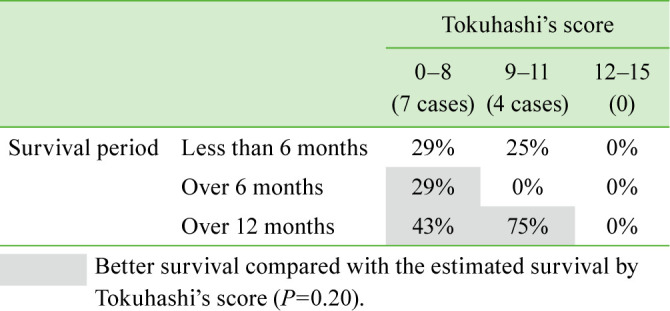
Activities of daily living were investigated using performance status3). Postoperative performance status at the last follow-up was compared with preoperative performance status. Paralysis was evaluated using the Frankel grade4). The actual survival after surgery was determined using data from clinical records and compared with the estimated survival after surgery calculated using the Tokuhashi score5). Better survival was defined as survival longer than the estimated survival calculated with the Tokuhashi score.
Statistical analyses
Chi-square analyses were performed to evaluate significant differences in improvement in paralysis and survival between the groups. Data were analyzed using IBM SPSS Statistics 24 software. P<0.05 was considered to indicate statistical significance.
Results
Clinical characteristics
For the 11 patients in the unknown tumor origin group, the initial symptoms included back pain (n=7), paralysis (n=2), gait disturbance caused by myelopathy (n=1), and muscle weakness of the arm (n=1). For the 15 patients in the known tumor origin group, the initial symptoms included neck or back pain (n=11), gait disturbance caused by myelopathy (n=2), paralysis (n=1), and numbness of the leg (n=1). The mean duration from initial symptom to paralysis in patients without initial paralysis was 1.9 months (range, 0.5–5.0 months) in the unknown tumor origin group and 2.3 months in the known tumor origin group (range, 0.5–7 months). The mean preoperative Tokuhashi score in the unknown tumor origin and known tumor origin groups was 5.8 (range, 2–10) and 6.0 (range, 1–9), respectively.
Clinical outcomes
Of the 11 patients with tumors of unknown origin, 6 underwent decompression with posterior instrumented fusion and 5 underwent posterior decompression alone without instrumentation. The mean operative time was 272 minutes (range, 98–605 minutes), and the mean estimated blood loss volume was 590 mL (range, 120–1,500 mL). By contrast, in the control group, 10 patients underwent decompression with posterior instrumented fusion, 3 underwent posterior fusion without decompression, and 2 underwent posterior decompression alone without instrumentation. The mean operative time in the control group was 271 minutes (range, 147–428 minutes), and the mean estimated blood loss was 607 mL (range, 100–4,100 mL). No significant differences in operative time or estimated blood loss were observed between the groups. The primary tumors in all the patients in the unknown tumor origin group were definitively diagnosed using tissue samples obtained during surgery and consisted of malignant lymphoma (n=4), lung cancer (n=2), prostate cancer (n=1), leukemia (n=1), renal pelvic cancer (n=1), liver cyst adenocarcinoma (n=1), and synovial sarcoma (n=1). The mean performance status score in the control group and the unknown tumor origin group was 3.6 and 3.6 preoperatively, which significantly improved to 2.7 and 2.9 postoperatively, respectively (P<0.05; Figure 1). Seven patients (64%) in the unknown tumor origin group showed improvement in paralysis by ≥1 Frankel grade (Table 3). By contrast, only 4 patients (27%) in the control group showed improvement in paralysis. The patients in the unknown tumor origin group tended to have better improvement in paralysis (P=0.05). All the patients with tumors of unknown origin underwent adjuvant therapy after definitive diagnosis following decompression and instrumented fusion surgery, which consisted of chemotherapy (n=9), radiotherapy (n=2), and hormonal therapy (n=1). The present series of patients with metastatic spinal tumors of unknown origin who underwent decompression and instrumented fusion surgery showed a slight tendency toward better survival than the estimated survival calculated using the Tokuhashi score (Table 4 ).
Figure 1.
Mean change in performance status.
Table 3. Change in the Frankel grade.
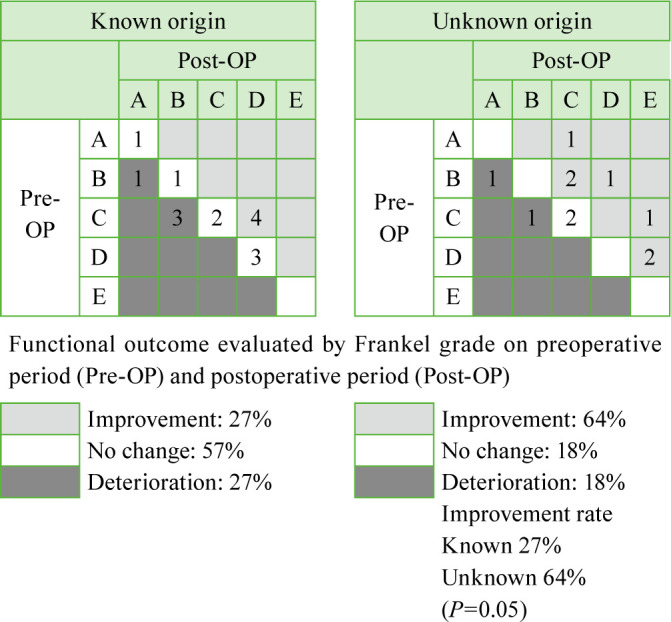
Table 4. Preoperative Tokuhashi’s score and survival period in the patient group with tumors of unknown origin.
Discussion
The present results demonstrate that emergent decompression/fusion surgery is potentially useful for diagnosing tumor origin and improving neurological outcomes and performance status. Moreover, the present results suggest that emergent decompression/fusion surgery might extend the survival of patients with paralysis caused by metastatic tumors of unknown origin.
Several possible reasons may explain the beneficial effects of emergent decompression/fusion surgery observed in the present series. One is a direct effect of decompression/fusion surgery. The surgery alone attenuated paralysis, possibly resulting in extended patient survival. In the present series, 64% of the patients with tumors of unknown origin showed significant postoperative improvement of neurological status. Paralysis itself might be a direct deteriorative factor impacting survival in patients with metastatic skeletal tumors5, 6). Recent studies have suggested that emergency surgery should be performed as soon as possible if the patient’s neurological state has deteriorated rapidly owing to metastatic spinal tumors7, 8). Moreover, better improvement of postoperative paralysis was significantly associated with longer survival9). Therefore, attenuation of paralysis is an important therapeutic objective for orthopedic/spinal surgeons in the multidisciplinary tumor team.
Another reason could be the fact that large samples for pathological analysis can be obtained during emergent decompression/fusion surgery, which may result in an increased chance for definitive pathological diagnosis as compared with needle biopsy, for which a diagnosis rate of 35–40% has been reported10, 11). In fact, all the patients in the present series were definitively diagnosed using tissue samples obtained during surgery.
Third, improvement in performance status achieved by decompression/fusion surgery enables the oncologist to administer adjuvant therapy12). In the present series, the patients showed postoperative improvement in performance status from 3.6 to 2.9. Thus, in this study, definitive pathological diagnosis and improvement of performance status by decompression/fusion surgery resulted in appropriate adjuvant therapy for patients with tumors of unknown origin.
The above-mentioned factors might have positively impacted the survival of the study patients. Although further analysis is needed to precisely evaluate its importance, emergent decompression/fusion surgery for paralysis caused by metastatic tumors of unknown origin appears to improve neurological outcomes and performance status and to potentially extend patient survival.
This research has some limitations. The sample size was relatively small, and the follow-up period was short. In addition, differences in neurological outcomes and performance status according to primary tumor site were not investigated. Further investigation could help clarify this information.
Conclusion
Emergent decompression/fusion surgery performed for patients with paralysis caused by metastatic tumors of unknown origin is potentially useful for diagnosing tumor origin and improving neurological outcomes and performance status, and thus for extending patient survival.
Conflicts of interest
The authors declare that they have no conflict of interest related to the present study.
References
- 1.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine 1990; 15: 1–4. doi: 10.1097/00007632-199001000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine 1985; 10: 19–20. doi: 10.1097/00007632-198501000-00003 [DOI] [PubMed] [Google Scholar]
- 3.Oken MM, Creech RH, Tormey DC. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 4.Frankel HL, Hancock DO, Hyslop G. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969; 7: 179–192. [DOI] [PubMed] [Google Scholar]
- 5.Tokuhashi Y, Uei H, Oshima M. Scoring system for prediction of metastatic spine tumor prognosis. World J Orthop 2014; 5: 262–271. doi: 10.5312/wjo.v5.i3.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol 2005; 6: 15–24. doi: 10.1016/S1470-2045(05)70022-X [DOI] [PubMed] [Google Scholar]
- 7.Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg Br 2010; 92: 1054–1060. doi: 10.1302/0301-620X.92B8.22296 [DOI] [PubMed] [Google Scholar]
- 8.Gasbarrini A, Li H, Cappuccio M. Efficacy evaluation of a new treatment algorithm for spinal metastases. Spine 2010; 35: 1466–1470. doi: 10.1097/BRS.0b013e3181c680b9 [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Chen HT, Hsu HC. Preoperative palsy score has no significant association with survival in non-small-cell lung cancer patients with spinal metastases who undergo spinal surgery. J Orthop Surg Res 2015; 10: 149. doi: 10.1186/s13018-015-0291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iizuka Y, Iizuka H, Tsutsumi S. Diagnosis of a previously unidentified primary site in patients with spinal metastasis: diagnostic usefulness of laboratory analysis, CT scanning and CT-guided biopsy. Eur Spine J 2009; 18: 1431–1435. doi: 10.1007/s00586-009-1061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. A prospective study of a diagnostic strategy. J Bone Joint Surg Am 1993; 75: 1276–1281. doi: 10.2106/00004623-199309000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Chong S, Shin SH, Yoo H. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients’ survival. Spine J 2012; 12: 1083–1092. doi: 10.1016/j.spinee.2012.10.015 [DOI] [PubMed] [Google Scholar]