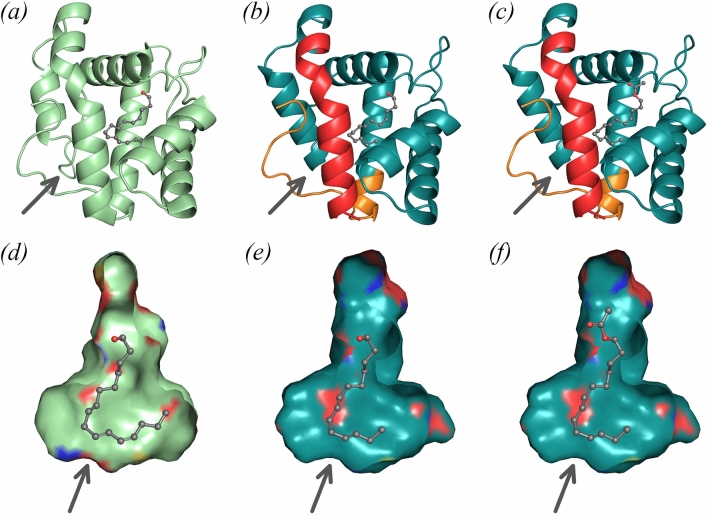
Figure 4.
Models for pheromone binding to EposPBP3. (a) Structure of BmorPBP1 bound to bombykol, (b) hybrid model of EposPBP3 with E11-14:OH and (c) hybrid model of EposPBP3 with E11-14:OAc. On (b,c), the experimentally-determined structure of EposPBP3 is shown in teal while the N- and C-termini built by homology modeling using the BmorPBP1 structure as template are shown in red and orange, respectively. (d) Internal cavity of BmorPBP1 with bound bombykol, (e) internal cavity of EposPBP3 in closed form with E11-14:OH and (f) internal cavity of EposPBP3 in closed form with E11-14:OAc. On (d–f), the coloured patches on the surfaces indicate the presence of oxygen (red), nitrogen (blue) and sulphur (yellow) atoms pointing towards the surface. Pheromone compounds are shown in sphere mode, with carbon and oxygens atoms coloured in grey and red, respectively. The arrows indicate the approximate location of the cavity opening shown on Fig. 3b. Image drawn using PyMOL Molecular Graphics System, Version 2.0 (https://pymol.org/2/).