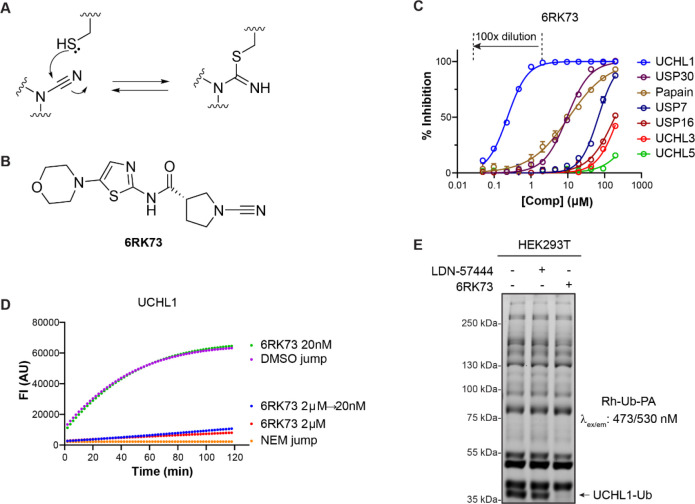
Figure 1.
Biochemical characterization of UCHL1 inhibitor 6RK73. (A) Reacting a thiol with a cyanimide results in the formation of an isothiourea adduct. (B) Structure of UCHL1 inhibitor 6RK73. (C) IC50 determination of 6RK73 for the indicated DUBs and papain. (D) Progress curves for UCHL1 proteolytic activity after jump dilution. (See also panel C.) DMSO and N-ethylmaleimide (NEM) are used as controls. (E) Fluorescence labeling of the remaining DUB activity in HEK293T cells upon treatment with UCHL1 inhibitors LDN-57444 and 6RK73.