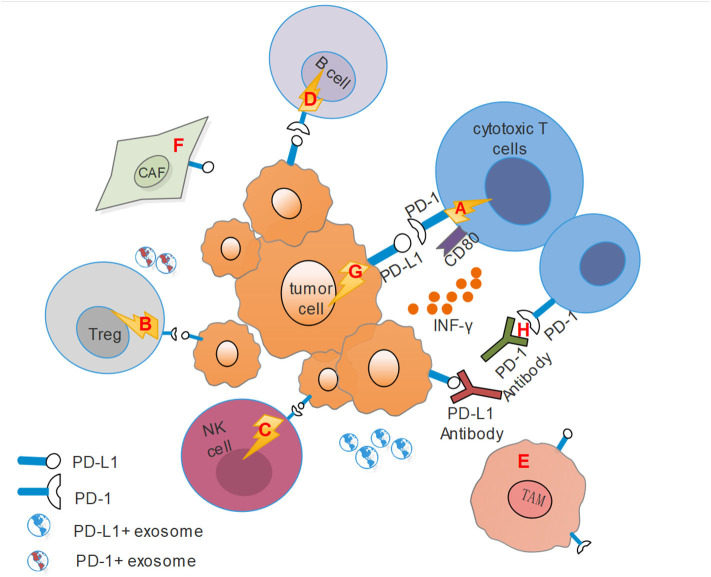
Figure 1.
This figure shows the immunosuppressive microenvironment mediated by PD-1/PD-L1 pathway. CAF, Cancer-associated fibroblasts; TAM, Tumor-associated macrophages; Treg, regulatory T cells. (A) PD-L1 on tumor cells combines with PD-1 on cytotoxic T cells transmits an inhibitory second signal to T cells, causing effector T cells exhaustion, dysfunction and tumor progression. At the same time, PD-L1 can act as a ligand to bind with CD80 on effector T cells, competitively inhibits the binding of costimulatory molecule CD28 with CD80, and hinders T cell activation. (B) PD-L1 also binds to PD-1 on Tregs, resulting in immune suppression by raising the threshold for T-cell activation. (C) For NK cells, PD-1 represents an “activated” phenotype and binds to PD-L1 on tumor cells or stromal cells, leading to its dysfunction. (D) Activation of PD-1 signals on B cells can inhibit the proliferation of CD4+ and CD8 T+ cells. (E) Activated CD4+ T helper cells modulated the up-regulation of PD-L1 expression on macrophages via IFN-γ, and TAMs could mediate adaptive resistance and dampen tumor specific T cell function based on PD-L1 expression. (F) HNSCC tumor cells recruit fibroblasts and up-regulate PD-L1 expression on fibroblasts. Conversely, fibroblasts can increase PD-L1 expression on HNSCC cells. (G) PD-L1 on tumor cells binding with PD-1 can transmit anti-apoptotic signals to the tumor themselves. (H) PD-1 antibodies can competitively inhibit the binding of PD-1 to PD-L1, while PD-L1 antibodies bind to PD-1, and they both inhibit the activation of the PD-1/PD-L1 signal pathway and reverse the suppressive effect.