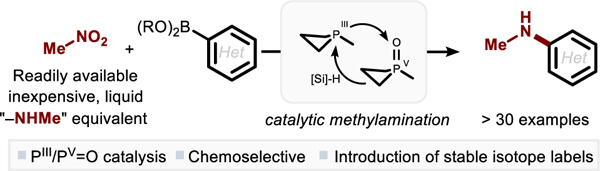
Abstract
The direct reductive N-arylation of nitromethane by organophosphorus-catalyzed reductive C–N coupling with arylboronic acid derivatives is reported. This method operates by the action of a small ring organophosphorus-based catalyst (1,2,2,3,4,4-hexamethylphosphetane P-oxide) together with a mild terminal reductant hydrosilane to drive the selective installation of the methylamino group to (hetero)aromatic boronic acids and esters. This method also provides for a unified synthetic approach to isotopically-labeled N-methylanilines from various stable isotopologues of nitromethane (i.e. CD3NO2, CH315NO2 and 13CH3NO2), revealing this easy-to-handle compound as a versatile precursor for the direct installation of the methylamino group.
Graphical Abstract
Nitromethane (H3C–NO2) is an industrially important commodity chemical, primarily used as a solvent, stabilizer, and fuel additive.1 Also, H3C–NO2 is a common and useful carbon pronucleophile in synthesis (Figure 1A, left),2–5 but it is comparatively less developed as an amination reagent. Recently, Jiao and coworkers reported H3C–NO2 as a precursor to “H2N–X” equivalents by reductive Nef-like decomposition,6 but an alternative reductive N-functionalization of H3C–NO2 that retains the “H3C–N” substructure would trace a role for this inexpensive and easily-handled liquid as a potential surrogate for methylamine—a gaseous List 1 controlled precursor7—especially in catalytic coupling chemistry (Figure 1A, right).8–11 At present, reductive N-functionalization of H3C–NO2 is limited to two isolated examples: Niggemann has reported reductive N-benzylation of nitromethane mediated by stoichiometric B2pin2 in the presence of excess BnZnBr,12 and Suarez-Pantiga and Sanz have reported a triphenylphosphine-mediated reductive N-phenylation of nitromethane catalyzed by an oxomolybdenum(VI) compound under microwave irradiation.13 We report here a general catalytic method for the methylamination of arylboronic acids and esters with H3C–NO2 by reductive C–N coupling driven by PIII/PV=O redox cycling (Figure 1B). These results expand the scope of reductive C–N coupling, generalize the use of H3C–NO2 as a methylamine surrogate in reductive cross coupling, and provide a unique pathway for introducing stable isotopes (15N, 13C, 2H) widely-used for metabolic tracing.14
Figure 1.
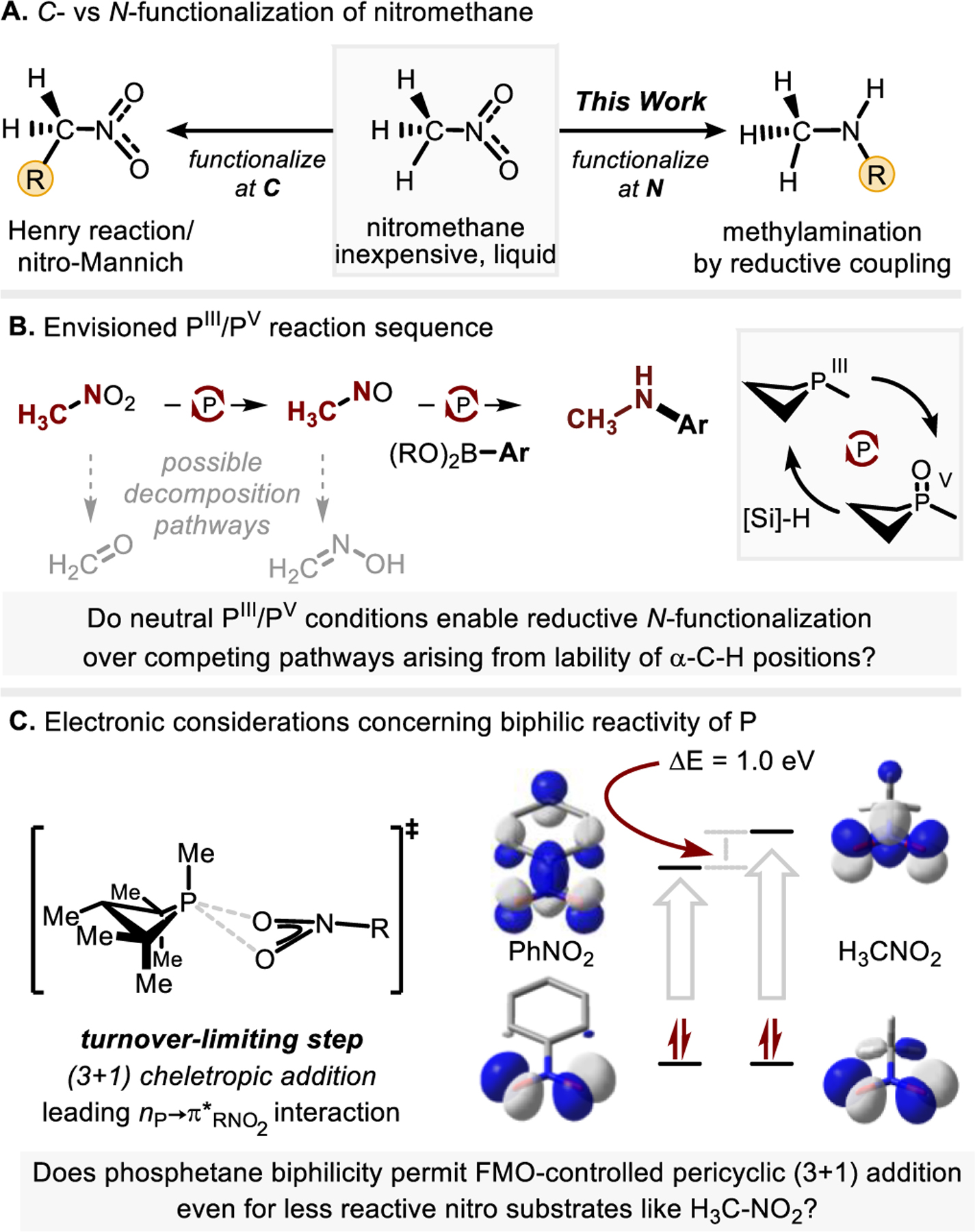
A) Uses of nitromethane in organic synthesis. B) Reaction sequence leading to formation of N-methylanilines via PIII/PV=O-catalyzed reductive C–N coupling of nitromethane with boronic acids (esters); C) Electronic challenge presented by high frontier orbital gap of H3C–NO2. FMO=frontier molecular orbital.
Prior work has established PIII/PV=O catalysis15–17 as a viable approach to reductive N-functionalization of nitroarene (Ar–NO2) substrates,18–20 but the translation of this technique to reductive N-functionalization of H3C–NO2 requires that the desired C–N coupling sequence (Figure 1B) outcompete numerous unproductive but well-known decomposition pathways, both for H3C–NO2 (e.g. Nef reaction) and potential deoxygenation intermediates (e.g. tautomerization of H3C–NO→H2C=NOH and H3C–N:→H2C=NH, inter alia). In principle, the base-free conditions of the PIII/PV=O catalyzed nitro functionalization, which depends on a rate-limiting (3+1) cheletropic addition of phosphine and nitro substrate, might suppress these processes. However, the nitro moiety of H3C–NO2 is inherently less reactive than Ar–NO2 as cycloaddition partner due to a larger local frontier orbital energy gap (ΔΔE= 1.0 eV, Figure 1C),21 resulting in less favorable pairwise orbital interactions that therefore impose a stringent demand of the biphilic character of the phosphetane catalyst.
Investigation of the reductive N-functionalization of H3C–NO2 was initiated with these considerations in mind. Empirical observations (Table 1) indicate that a catalytic system comprising the organophosphorus O-atom transfer catalyst 1•[O]22 and a terminal hydrosilane reductant indeed successfully achieve a methylamination of arylboronic acids by PIII/PV=O-catalyzed intermolecular C–N coupling. With 4-fluorophenylboronic acid (3) as a representative substrate, the reductive functionalization of 1.0 equiv of commercial reagent grade H3C–NO2 (2) by 10 mol% of 1•[O] and 2 equiv of PhSiH3 gave a promising 35% yield of N-methyl-4-fluoroaniline (4) (Table 1, entry 1), but simply increasing equivalencies of inexpensive H3C–NO2 (3.0 equiv, entry 2) resulted in excellent yield of 4 (95% NMR yield, 90% isolated yield). A high yield was similarly obtained with phosphetane 1 as a precatalyst (entry 3)—consistent with PIII/PV=O redox cycling—but omission of either the precatalyst 1•[O] or phenylsilane failed to provide methylamination product 4 (entries 4–5). Inexpensive and bench-stable polymethylhydrosiloxane (PMHS) is also a viable terminal reductant for the reaction, albeit with somewhat lower efficiency (85%, entry 6). In situ spectral monitoring of the reductive C−N coupling showed that isotopically enriched nitromethane-d3 (δ 4.32 ppm) is cleanly converted to the product N-(methyl-d3)-4-bromoaniline (δ 2.79 ppm), and no side products or long-lived intermediates were observed (Figure S2). The relatively high concentration of the PIII catalyst resting state 1 presumably drives the methylamination forward along the productive reaction pathway in preference to possible side pathways, including the favorable isomerization of nitrosomethane to formaldoxime.23,24
Table 1.
Discovery and Control Experiments for Organophosphorus-Catalyzed N-methylaniline Synthesis.a
 | |||
|---|---|---|---|
| Entry | silane | R3P=O | Yield (%)b |
| 1c | PhSiH3 | 1•[O] | 35 |
| 2 | PhSiH3 | 1•[O] | 95 (90)d |
| 3 | PhSiH3 | 1 | 94 |
| 4 | PhSiH3 | None | 0 |
| 5 | None | 1•[O] | 0 |
| 6e | PMHS | 1•[O] | 85 |
Unless otherwise noted, reactions were carried out with 1•[O] (10 mol %), phenylsilane (0.5 mmol, 2.0 equiv), 4-fluorophenylboronic acid (0.25 mmol) and nitromethane (0.75 mmol, 3.0 equiv) in CPME (0.5 mL) at 120 °C for 18 h.
Yields were determined by 19F NMR integration with the aid of an internal standard.
1.0 equiv of 2 was used.
Isolated yield.
24 h reaction time.
As depicted in Figure 2A, the PIII/PV=O-catalyzed methylamination method shows excellent functional group tolerance, several facets of which provide for chemoselectivities that complement transition-metal catalyzed C–N coupling methods. For example, halogen substituents are well-tolerated (5a, 8–10, 16) in the PIII/PV=O-catalyzed methylamination and intermolecular C–N coupling occurs exclusively at the boronic acid position. Also, the orthogonal reactivity to the nitro group with respect to other nitrogen-containing functional groups permits selective aryl methylamination even in the presence of free −NH2 groups (16) without explicit protection. Carbonyl functionalities such esters (13) and ketones (17, 19) that might be susceptible to acylation or condensation reaction with H3C–NH2 are retained when H3C–NO2 is used as a surrogate. Furthermore, substrates with other functional groups including benzyloxy (7), tetrahydropyranyl ether (14), thiomethyl (15), and trifluoromethyl (18) groups all yielded the corresponding N-methylaniline products in good yields. The method is also applicable to compounds with known bioactive core structures, such as derivatives of celecoxib (18), estrone (19), and Pittsburgh compound B25 (20).
Figure 2.
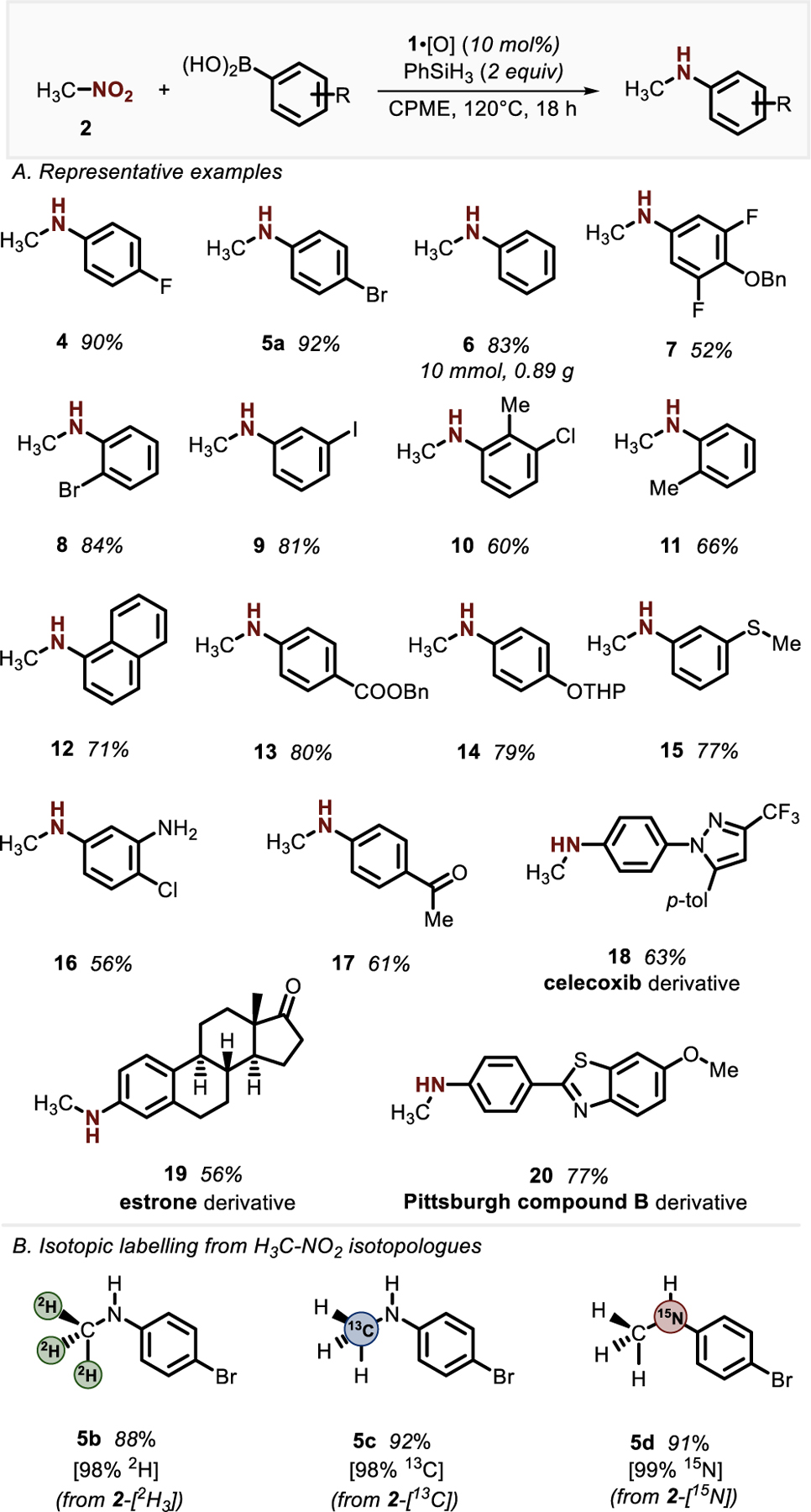
Synthetic scope of PIII/PV=O-catalyzed C–N coupling of nitromethane and arylboronic acids. See SI for full experimental details and conditions. Yields are reported for material obtained following purification and isolation.
The ability to use of H3C–NO2 as a simple synthetic precursor for the MeHN– group presents the opportunity for the preparation of diverse stable-isotope labeled products—useful tools in both laboratory26 and pharmaceutical27 research—through a unified synthetic strategy. As illustrated in Figure 2B, a suite of isotopically labeled N-methylaniline products 5b–5d are accessible with programmed labeling as dictated by the initial isotopic composition of the nitromethane isotopologue (viz. 2H3C–NO2 (2b), H313C–NO2 (2c), H3C–15NO2 (2d)). The relative ease with which the isotopologues of nitromethane are accessed make this an attractive approach to potentially valuable stable isotope labeled compounds.
Initial attempts to directly transfer the foregoing PIII/PV=O-catalyzed conditions to methylamination of 3-quinolylboronic acid resulted in a disappointing 19% yield of the target compound 21 (Figure 3A, yield in parenthesis). Studies showed that protodeboronation of the heteroarylboronic acid was a primary pathway limiting the productive C–N coupling in this case.28 Correspondingly, PIII/PV=O-catalyzed methylamination of 3-quinolylboronic acid 1,3-propanediolate ester showed a significant improvement in C–N coupling yield (93%), in line with the relative stability of boronic esters to protodeboronation as compared to their parent boronic acids.27 This phenomenon is general; B-heteroaryl-1,3,2-dioxaborinanes including a variety of five- and six-membered heterocyclic derivatives, such as isoquinoline (22), indazole (23) pyridine (24, 27), thiophene (25) and pyrimidine (26), were successfully transformed to the corresponding N-methylamine derivatives with improved yields relative to their arylboronic acid congeners. B-Aryl-1,3,2-dioxaborinanes containing electron-donating (28) or electron-withdrawing group (29) transform smoothly to the desired products, showing the generality of utilizing boronate esters for methylamination reactions. Other common boronate ester residues such as a neopentyl glycol ester (e.g. Ar-Bnep 30, Figure 3B) and pinacol esters (e.g. Ar-Bpin 31–33, Figure 3C) gave similar efficiency in this methylamination process. Notably, subjection of benzene-1,4-diboronic acid bispinacol ester to the standard reaction conditions afforded single methylamination product 31 with excellent yield; evidently the second (pinacolato)boryl moiety becomes electronically deactivated for amination following an initial reductive C−N coupling.
Figure 3.
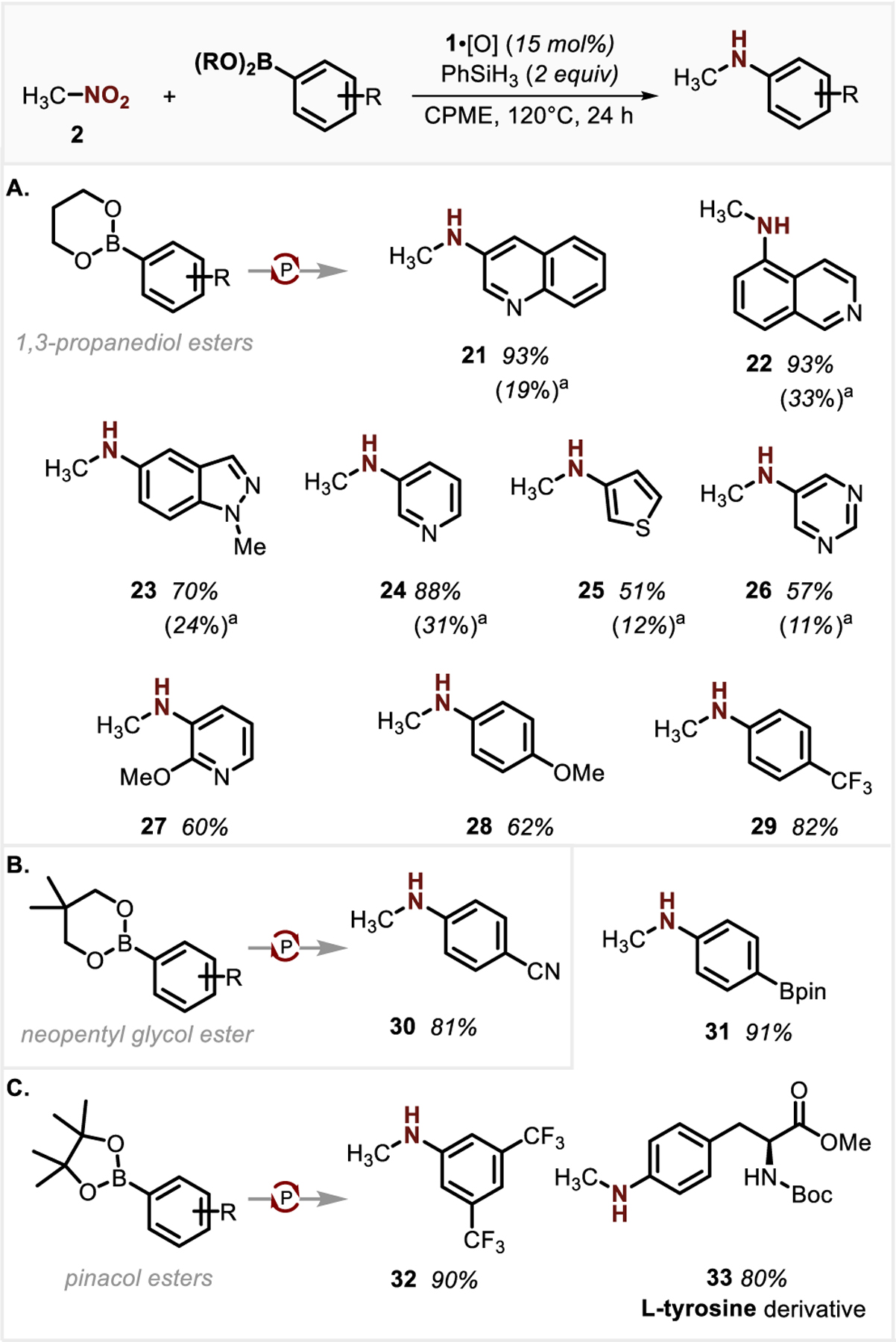
Synthetic scope of PIII/PV=O-catalyzed C–N coupling of nitromethane and (hetero)arylboronic esters. See SI for full experimental details and conditions. Yields are reported for material obtained following purification and isolation. a Yields for reactions with boronic acids were determined by 1H NMR with dibromomethane as the internal standard.
Competition experiments evidence a differential reactivity of H3C–NO2 as compared to Ar–NO2 substrates in PIII/PV=O-catalyzed reductive C–N coupling. As illustrated in Figure 4A, an equimolar mixture of four componenets—namely H3C–NO2 (2), 4-NC-C6H4–NO2 (34), 3,5-(F3C)2-C6H3–Bpin (35) and 4-H3CO-C6H4–B(OH)2 (36)—was subjected to standard reductive C–N coupling conditions catalyzed by 1•[O] in a one pot manner. In the event, only two of the possible four products—specifically methylaniline 29 and diarylamine 37—were observed in greater than 5% yield. Consistent with the FMO rationale in Figure 1,15i this observation implies that nitroarene 34 reacts more quickly than nitromethane 2 via (3+1) cheletropic addition with 1, and goes on to couple selectively with the more reactive boronic acid partner 36. In a subsequent event, H3C–NO2 then reacts to give 29 by methylamination of the more inert boronic ester 35, proceeding without diminished yield for these reaction partners.29 This kinetically-controlled chemoselectivity was further demonstrated by the modular synthesis of N,N’-difunctionalized phenylenediamine (Figure 4B, 42). The three-component coupling of H3C–NO2, Ph–B(OH)2 (40), and 4-nitrophenylboronic ester (41) produced 42 via sequential amination reactions with almost complete suppression of undesired methylaminated product (6).
Figure 4.
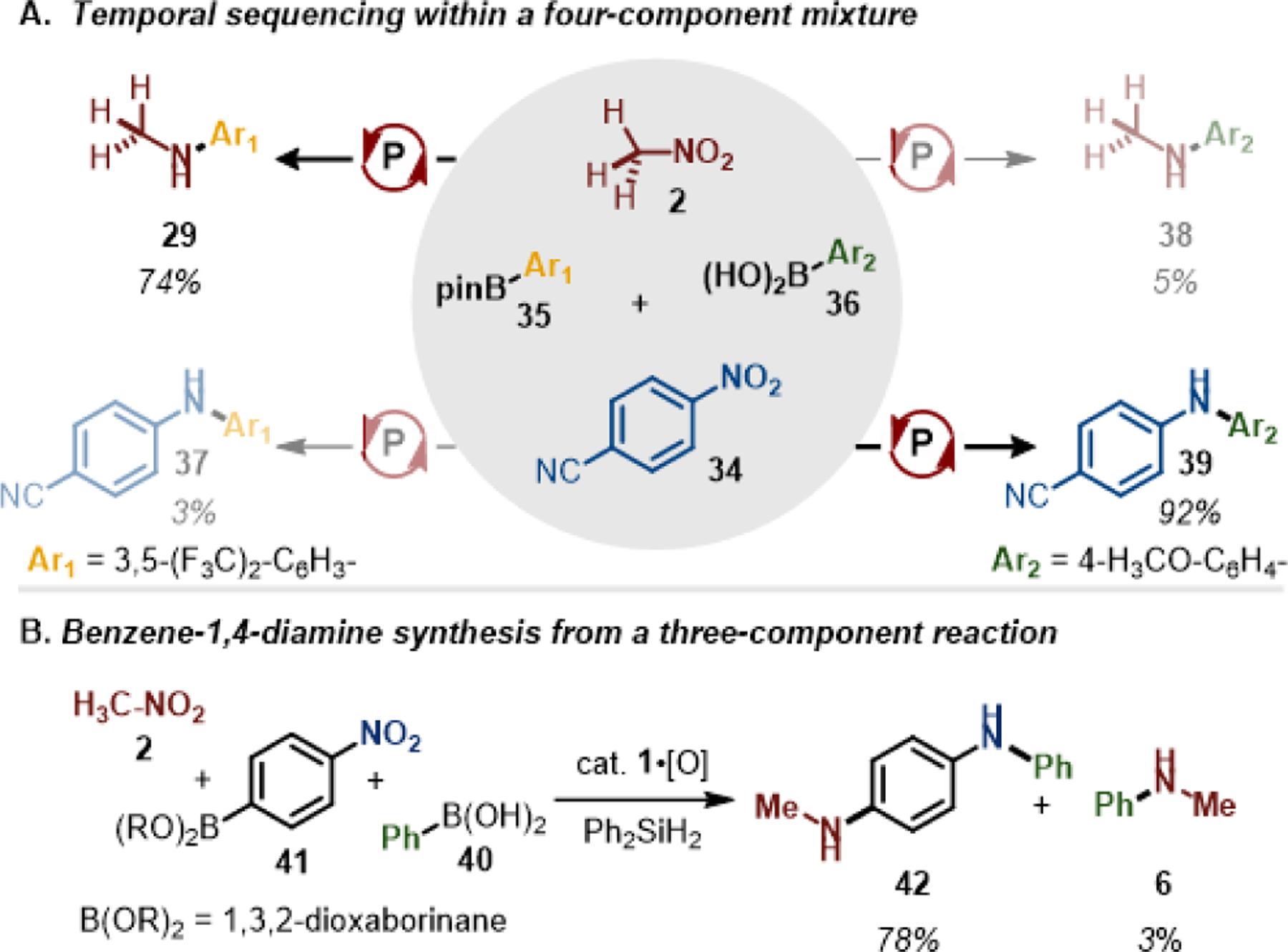
A) Reaction of a four component mixture illustrating differential reactivity of H3CNO2 vs ArNO2 and ArB(OH)2 vs ArB(OR)2. (B) Selective three-component coupling via sequential C–N coupling reactions.
A synthesis of the anxiolytic triflubazam30 (45, Figure 5) contextualizes the reductive C–N coupling of nitromethane within the growing portfolio of PIII/PV-catalyzed methods promoted by phosphetane catalyst 1•[O]. Specifically, PIII/PV-catalyzed reductive C–N coupling of phenylboronic acid and 4,4,5,5-tetramethyl-2-(2-nitro-5-(trifluoromethyl)phenyl)-1,3,2-dioxaborolane (43), followed in situ by PIII/PV-catalyzed amidation15h,31 with monoethyl malonate gives diarylamide 44 in a one-pot sequence. Subsequent PIII/PV-catalyzed methylamination by C–N coupling with H3C–NO2 installs the MeHN– moiety and induces intramolecular cyclization to close the diazepinedione ring, furnishing the medicinal target 45 in an overall 51% yield for the two-pot, three-step, all-PIII/PV-catalyzed sequence.
Figure 5.
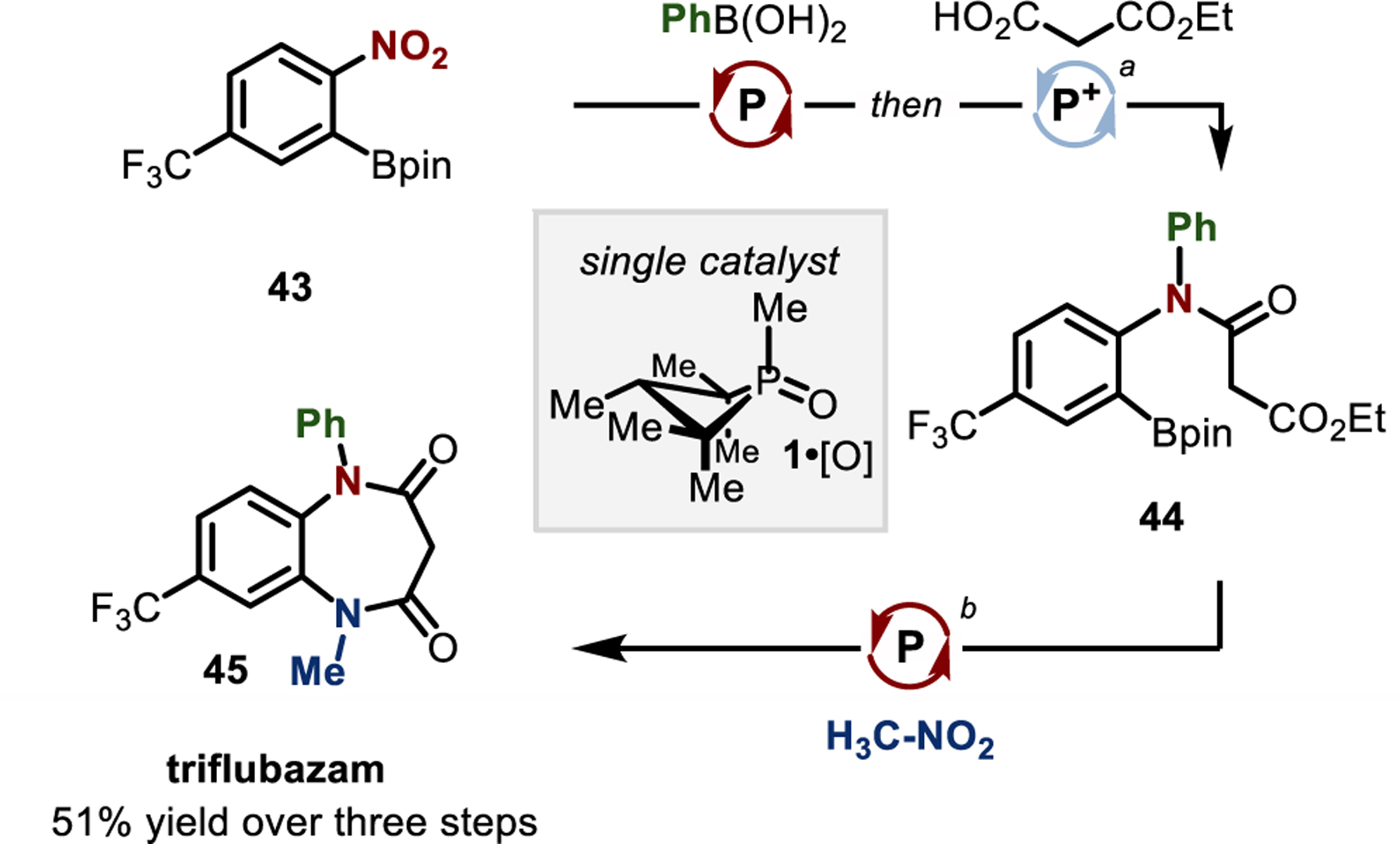
Two-pot, three step synthesis of triflubazam by an all-PIII/PV-catalyzed sequence. Reaction conditions: (a) 43 (1.0 equiv), PhB(OH)2 (1.1 equiv), Ph2SiH2 (4.0 equiv), 1•[O] (30 mol%), CPME, 120 °C ; then add monoethyl malonate (1.2 equiv), diethylbromomalonate (1.5 equiv), 40 °C; (b) 44 (1.0 equiv), H3C–NO2 (3.0 equiv), Ph2SiH2 (3.0 equiv), 1•[O] (15 mol%), CPME, 120 °C.
In summary, we have demonstrated that nitromethane is an inexpensive and easy-to-handle synthetic equivalent for installation of the MeHN– fragment via PIII/PV=O catalysis. Readily-available boronic acids and esters are selectively methylaminated in the presence of various functional groups and heteroaromatics. The method serves as a robust complementary tactic to transition metal catalyzed C–N coupling techniques relying on the use of MeNH2 or related surrogates. With respect to our efforts to develop PIII/PV=O-catalyzed reductive C–N coupling reactions as modular approach to complex amine synthesis, this study represents our first success with nitroalkane substrates and portends future developments in the deoxygenative N-functionalization of higher nitroalkane homologues to access more elaborate N-alkyl products
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by NIH NIGMS (GM114547). The authors thank Prof. A. Wendlandt (MIT) and Drs. J.M. Lipshultz, T.V. Nykaza and J.C. Cooper for helpful discussion.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
General methods and synthetic procedures (.pdf)
1H, 2H, 13C, 15N, 19F and 31P NMR spectra (.pdf).
The authors declare no competing financial interests.
REFERENCE
- 1.Markofsky SB Nitro Compounds, Aliphatic In Ullmann’s Encyclopedia of Industrial Chemistry, 7th edition; Ley C, Ed.; Wiley-VCH: Weinheim, 2012; Vol. 24, pp 291–300. [Google Scholar]
- 2.(a) Ono N The Nitro-aldol (Henry) Reaction In The Nitro Group in Organic Synthesis; Feuer H, Ed.; Wiley-VCH: New York, 2001, pp 30–69; [Google Scholar]; (b) Luzzio FA The Henry Reaction: Recent Examples. Tetrahedron 2001, 57, 915–945. [Google Scholar]
- 3.Noble A; Anderson JC Nitro-Mannich Reaction. Chem. Rev 2013, 113, 2887–2939. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ballini R; Bosica G; Fiorini D; Palmieri A; Petrini M Conjugate Additions of Nitroalkanes to Electron-Poor Alkenes: Recent Results. Chem. Rev 2005, 105, 933–971. [DOI] [PubMed] [Google Scholar]; (b) Manzano R; Andrés JM; Álvarez R; Muruzábal MD; De Lera ÁR; Pedrosa R Enantioselective Conjugate Addition of Nitro Compounds to α,β-Unsaturated Ketones: An Experimental and Computational Study. Chem. Eur. J 2011, 17, 5931–5938. [DOI] [PubMed] [Google Scholar]; (c) Ballini R; Palmieri A Formation of Carbon-Carbon Double Bonds: Recent Developments via Nitrous Acid Elimination (NAE) from Aliphatic Nitro Compounds. Adv. Synth. Catal 2019, 361, 5070–5097. [Google Scholar]
- 5.(a) Li CJ; Li Z Green Chemistry: The Development of Cross-Dehydrogenative Coupling (CDC) for Chemical Synthesis. Pure Appl. Chem 2006, 78, 935–945. [Google Scholar]; (b) Li CJ Cross-Dehydrogenative Coupling (CDC): Exploring C–C Bond Formations beyond Functional Group Transformations. Acc. Chem. Res 2009, 42, 335–344. [DOI] [PubMed] [Google Scholar]; (c) Girard SA; Knauber T; Li CJ The Cross-Dehydrogenative Coupling of Csp3 -H Bonds: A Versatile Strategy for C–C Bond Formations. Angew. Chem. Int. Ed 2014, 53, 74–100. [DOI] [PubMed] [Google Scholar]; (d) Varun BV; Dhineshkumar J; Bettadapur KR; Siddaraju Y; Alagiri K; Prabhu KR Recent Advancements in Dehydrogenative Cross Coupling Reactions for C–C Bond Formation. Tetrahedron Lett 2017, 58, 803–824. [Google Scholar]
- 6.Liu J; Zhang C; Zhang Z; Wen X; Dou X; Wei J; Qiu X; Song S; Jiao N Nitromethane as a Nitrogen Donor in Schmidt-Type Formation of Amides and Nitriles. Science 2020, 367, 281–285. [DOI] [PubMed] [Google Scholar]
- 7.Records and Reports of Listed Chemicals and Certain Machines; Importation and Exportation of Certain Machines. Code of Federal Regulations Part 1310, Title 21, (2019).
- 8.Hughes EC; Veatch F; Elersich V N-Methylaniline From Chlorobenzene and Methylamine. Ind. Eng. Chem 1950, 42, 787–790. [Google Scholar]
- 9. Coupling with methylamine solutions:; (a) Fors BP; Watson DA; Biscoe MR; Buchwald SL A Highly Active Catalyst for Pd-Catalyzed Amination Reactions: Cross-Coupling Reactions Using Aryl Mesylates and the Highly Selective Monoarylation of Primary Amines Using Aryl Chlorides. J. Am. Chem. Soc 2008, 130, 13552–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Henderson JL; Buchwald SL Efficient Pd-Catalyzed Amination Reactions for Heterocycle Functionalization. Org. Lett 2010, 12, 4442–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fors BP; Buchwald SL A Multiligand Based Pd Catalyst for C–N Cross-Coupling Reactions. J. Am. Chem. Soc 2010, 132, 15914–15917. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Crawford SM; Lavery CB; Stradiotto M BippyPhos: A Single Ligand with Unprecedented Scope in the Buchwald-Hartwig Amination of (Hetero)Aryl Chlorides. Chem. Eur. J 2013, 19, 16760–16771. [DOI] [PubMed] [Google Scholar]; Ullman C–N Cross Coupling:; (e) Jiao J; Zhang XR; Chang NH; Wang J; Wei JF; Shi XY; Chen ZG A Facile and Practical Copper Powder-Catalyzed, Organic Solvent-and Ligand-Free Ullmann Amination of Aryl Halides. J. Org. Chem 2011, 76, 1180–1183. [DOI] [PubMed] [Google Scholar]; (f) Siddegowda MS; Yathirajan HS; Ramakrishna RA A Ligand-Free and Base-Free Copper Catalyzed Reaction: Arylation of Ammonia and Primary Amines as Their Acetate Salts. Tetrahedron Lett 2012, 53, 5219–5222. [Google Scholar]; (g) Kumar AS; Ramani T; Sreedhar B Magnetically Separable CuFe2O4 Nanoparticles in PEG: A Recyclable Catalytic System for the Amination of Aryl Iodides. Synlett 2013, 24, 938–942. [Google Scholar]; (h) Wang D; Kuang D; Zhang F; Yang C; Zhu X Room-Temperature Copper-Catalyzed Arylation of Dimethylamine and Methylamine in Neat Water. Adv. Synth. Catal 2015, 357, 714–718. [Google Scholar]
- 10. Coupling with methylammonium salts:; (a) Green RA; Hartwig JF Palladium-Catalyzed Amination of Aryl Chlorides and Bromides with Ammonium Salts. Org. Lett 2014, 16, 4388–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Green RA; Hartwig JF Nickel-Catalyzed Amination of Aryl Chlorides with Ammonia or Ammonium Salts. Angew. Chem. Int. Ed 2015, 54, 3768–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zeng L; Fu H; Qiao R; Jiang Y; Zhao Y Efficient Copper-Catalyzed Synthesis of N-Alkylanthranilic Acids via an Ortho-Substituent Effect of the Carboxyl Group of 2-Halobenzoic Acids at Room Temperature. Adv. Synth. Catal 2009, 351, 1671–1676. [Google Scholar]
- 11.Coupling with protected methylamine surrogates:; (a) Trabanco AA; Vega JA; Fernandez MA Fluorous-Tagged Carbamates for the Pd-Catalyzed Amination of Aryl Halides. J. Org. Chem 2007, 72, 8146–8148. [DOI] [PubMed] [Google Scholar]; (b) Bernardi P; Dembech P; Fabbri G; Ricci A; Seconi G A General and Convenient Procedure for the Synthesis of N- Alkylarylamines and N-Alkylheteroarylamines by Electrophilic Amination of Cuprates with N-Alkylhydroxylamines. J. Org. Chem 1999, 64, 641–643. [Google Scholar]
- 12.Rauser M; Ascheberg C; Niggemann M Direct Reductive N-Functionalization of Aliphatic Nitro Compounds. Chem. Eur. J 2018, 24, 3970–3974. [DOI] [PubMed] [Google Scholar]
- 13.Suárez-Pantiga S; Hernández-Ruiz R; Virumbrales C; Pedrosa MR; Sanz R Reductive Molybdenum-Catalyzed Direct Amination of Boronic Acids with Nitro Compounds. Angew. Chem. Int. Ed 2019, 58, 2129–2133. [DOI] [PubMed] [Google Scholar]
- 14.(a) Harbeson SL; Tung RD Deuterium in Drug Discovery and Development. Annu. Rep. Med. Chem 2011, 46, 403–417. [Google Scholar]; For examples highlighting the importance of isotope labeling:; (b) Atzrodt J; Derdau V Pd− and Pt− catalyzed H/D exchange methods and their application for internal MS standard preparation from a Sanofi-Aventis perspective J. Labelled Compd. Radiopharm 2010, 53, 674–685. [Google Scholar]; (c) Gant TGJ Using Deuterium in Drug Discovery: Leaving the Label in the Drug. J. Med. Chem 2014, 57, 3595–3611. [DOI] [PubMed] [Google Scholar]; (d) Atzrodt J; Derdau V; Kerr WJ; Reid M Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed 2018, 57, 1758–1784. [DOI] [PubMed] [Google Scholar]; (e) Zachleder V; Vítová M; Hlavová M; íková Š; Mojzeš P; Heumann H; Becher JR; Bišová K Stable isotope compounds - production, detection, and application. Biotechnol. Adv 2018, 36, 784–797. [DOI] [PubMed] [Google Scholar]
- 15.(a) Reichl KD; Dunn NL; Fastuca NJ; Radosevich AT Biphilic Organophosphorus Catalysis: Regioselective Reductive Transposition of Allylic Bromides via PIII/PV Redox Cycling. J. Am. Chem. Soc 2015, 137, 5292–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao W; Yan PK; Radosevich AT A Phosphetane Catalyzes Deoxygenative Condensation of α-Keto Esters and Carboxylic Acids via PIII/PV═O Redox Cycling. J. Am. Chem. Soc 2015, 137, 616–619. [DOI] [PubMed] [Google Scholar]; (c) Lin Y-C; Hatzakis E; McCarthy SM; Reichl KD; Lai T-Y; Yennawar HP; Radosevich AT P–N Cooperative Borane Activation and Catalytic Hydroboration by a Distorted Phosphorous Triamide Platform. J. Am. Chem. Soc 2017, 139, 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nykaza TV; Harrison TS; Ghosh A; Putnik RA; Radosevich AT A Biphilic Phosphetane Catalyzes N–N Bond-Forming Cadogan Heterocyclization via PIII/PV═O Redox Cycling. J. Am. Chem. Soc 2017, 139, 6839–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nykaza TV; Ramirez A; Harrison TS; Luzung MR; Radosevich AT Biphilic Organophosphorus-Catalyzed Intramolecular Csp2–H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc 2018, 140, 3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nykaza TV; Cooper JC; Li G; Mahieu N; Ramirez A; Luzung MR; Radosevich AT Intermolecular Reductive C–N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc 2018, 140, 15200–15205. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ghosh A; Lecomte M; Kim-Lee S-H; Radosevich AT Organophosphorus-Catalyzed Deoxygenation of Sulfonyl Chlorides: Electrophilic (Fluoroalkyl)Sulfenylation by PIII/PV=O Redox Cycling. Angew. Chem. Int. Ed 2019, 58, 2864–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lecomte M; Lipshultz JM; Kim-Lee S-H; Li G; Radosevich AT Driving Recursive Dehydration by PIII/PV Catalysis: Annulation of Amines and Carboxylic Acids by Sequential C–N and C–C Bond Formation. J. Am. Chem. Soc 2019. 141, 12507–12512. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Li G; Nykaza TV; Cooper JC; Ramirez A; Luzung MR; Radosevich AT An Improved PIII/PV═O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc 2020, 142, 6786. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Nykaza TV; Li G; Yang J; Luzung MR; Radosevich AT PIII/PV=O-Catalyzed Cascade Synthesis of N-Functionalized Azaheterocycles. Angew. Chem. Int. Ed 2020, 59, 4505–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. For a review of PIII/PV=O redox cycling, see:; (a) Marsden SP Catalytic Variants of Phosphine Oxide-Mediated Organic Transformations in Sustainable Catalysis; Dunn PJ, Hii KK, Krische MJ, Williams MT, Eds.; John Wiley & Sons, Inc.: New York, 2013; pp 339–361. [Google Scholar]; (b) Guo H; Fan YC; Sun Z; Wu Y; Kwon O Phosphine Organocatalysis. Chem. Rev 2018, 118, 10049–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. For recent examples of PIII/PV=O redox cycling, see:; (a) O’Brien CJ; Tellez JL; Nixon ZS; Kang LJ; Carter AL; Kunkel SR; Przeworski KC; Chass GA Recycling the Waste: The Development of a Catalytic Wittig Reaction Angew. Chem. Int. Ed 2009, 48, 6836–6839. [DOI] [PubMed] [Google Scholar]; (b) O’Brien CJ; Lavigne F; Coyle EE; Holohan AJ; Doonan BJ Breaking the Ring through a Room Temperature Catalytic Wittig Reaction Chem. Eur. J 2013, 19, 5854–5858. [DOI] [PubMed] [Google Scholar]; (c) O’Brien CJ; Nixon ZS; Holohan AJ; Kunkel SR; Tellez JL; Doonan BJ; Coyle EE; Lavigne F; Kang LJ; Przeworski KC Part I: The Development of the Catalytic Wittig Reaction Chem. Eur. J 2013, 19, 15281–15289. [DOI] [PubMed] [Google Scholar]; (d) Coyle EE; Doonan BJ; Holohan AJ; Walsh KA; Lavigne F; Krenske EH; O’Brien CJ Catalytic Wittig Reactions of Semi- and Nonstabilized Ylides Enabledby Ylide Tuning Angew. Chem. Int. Ed 2014, 53, 12907–12911. [DOI] [PubMed] [Google Scholar]; (e) van Kalkeren HA; Leenders SHAM; Hommersom CRA; Rutjes FPJT; van Delft FL In Situ Phosphine Oxide Reduction: A Catalytic Appel Reaction Chem. Eur. J 2011, 17, 11290–11295. [DOI] [PubMed] [Google Scholar]; (f) van Kalkeren HA; Bruins JJ; Rutjes FPJT; van Delft FL Organophosphorus-Catalysed Staudinger Reduction Adv. Synth. Catal 2012, 354, 1417–1421. [Google Scholar]; (g) Lee C; Chang T; Yu J; Reddy GM; Hsiao M; Lin W Synthesis of functionalized furans via chemoselective reduction/Wittig reaction using catalytic triethylamine and phosphine. Org. Lett 2016, 18, 3758–3761. [DOI] [PubMed] [Google Scholar]; (h) Saleh N; Voituriez A Synthesis of 9H-pyrrolo[1,2-a]indole and 3H-pyrrolizine derivatives via a phosphine-catalyzed umpolung addition/intramolecular Wittig reaction. J. Org. Chem 2016, 81, 4371–4377. [DOI] [PubMed] [Google Scholar]; (i) Saleh N; Blanchard F; Voituriez A Synthesis of nitrogen containing heterocycles and cyclopentenone derivatives via phosphine catalyzed Michael addition/intramolecular Wittig reaction. Adv. Synth. Catal 2017, 359, 2304–2315. [Google Scholar]; (j) Zhang K; Cai L; Yang Z; Houk KN; Kwon O Bridged [2.2.1] bicyclic phosphine oxide facilitates catalytic γ-umpolung addition−Wittig olefination. Chem. Sci 2018, 9, 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Cai L; Zhang K; Chen S; Lepage RJ; Houk KN; Krenske EH; Kwon O Catalytic Asymmetric Staudinger–Aza-Wittig Reaction for the Synthesis of Heterocyclic Amines. J. Am. Chem. Soc 2019, 141, 9537–9542. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Lorton C; Castanheiro T; Voituriez A Catalytic and Asymmetric Process via PIII/PV═O Redox Cycling: Access to (Trifluoromethyl)Cyclobutenes via a Michael Addition/Wittig Olefination Reaction. J. Am. Chem. Soc 2019, 141, 10142–10147. [DOI] [PubMed] [Google Scholar]
- 18.(a) Sapountzis I; Knochel P A New General Preparation of Polyfunctional Diarylamines by the Addition of Functionalized Arylmagnesium Compounds to Nitroarenes. J. Am. Chem. Soc 2002, 124, 9390–9391. [DOI] [PubMed] [Google Scholar]; (b) Doyle W; Staubitz A; Knochel P Mild Synthesis of Polyfunctional Benzimidazoles and Indoles by the Reduction of Functionalized Nitroarenes with Phenylmagnesium Chloride. Chem. Eur. J 2003, 9, 5323–5331. [DOI] [PubMed] [Google Scholar]; (c) Kopp F; Sapountzis I; Knochel P Preparation of Polyfunctionalized Amines by the Addition of Functionalized Organomagnesium Reagents to Nitrosoarenes Synlett, 2003, 885–887.; (d) Sapountzis I; Knochel P A New Method for the Selective Amination of 1,3- and 1,4-Dinitrobenzenes and Protected Nitroanilines Leading to Polyfunctional 1,3- and 1,4- Disubstituted Anilines. Synlett 2004, 955–958.; (e) Dhayalan V; Saemann C; Knochel P Synthesis of polyfunctional secondary amines by the addition of functionalized zinc reagents to nitrosoarenes. Chem. Commun 2015, 51, 3239–3242. [DOI] [PubMed] [Google Scholar]; (f) Gao H; Xu Q-L; Ess DH; Kürti L Transition-Metal-Free, Low-Temperature Intramolecular Amination of Aromatic C-H Bonds: Rapid Synthesis of Fused Heterocycles. Angew. Chem. Int. Ed 2014, 53, 2701–2705. [DOI] [PubMed] [Google Scholar]; (g) Rauser M; Ascheberg C; Niggemann M Electrophilic Amination with Nitroarenes. Angew. Chem. Int. Ed 2017, 56, 11570–11574. [DOI] [PubMed] [Google Scholar]
- 19.(a) Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886–891. [DOI] [PubMed] [Google Scholar]; (b) Cheung CW; Hu X Amine synthesis via iron-catalysed reductive coupling of nitroarenes with alkyl halides. Nat. Commun 2016, 7, 12494. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cheung CW; Hu X Nickel-Catalyzed Reductive Transamidation of Secondary Amides with Nitroarenes. ACS Catal 2017, 7, 7092–7096. [Google Scholar]
- 20.Xiao J; He Y; Ye F; Zhu S Remote Sp3 C–H Amination of Alkenes with Nitroarenes. Chem 2018, 4, 1645–1657. [Google Scholar]
- 21.See SI for Kohn-Sham frontier orbital eigenvalues for H3C-NO2 and H5C6-NO2 at the ωB97XD/6–311++G(2d,2p) level of theory.
- 22.For a preparation of 1•[O], see: Nykaza TV; Cooper JC; Radosevich AT anti-1,2,2,3,4,4-Hexamethylphosphetane 1-Oxide. Org. Synth 2019, 96, 418–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Adeney PD; Bouma WJ; Radom L; Rodwell WR Nitrosomethane and Its Nitrone and Oxime Isomers. A Theoretical Study of 1,2- and 1,3-Intramolecular Hydrogen Shifts. J. Am. Chem. Soc 1980, 102, 4069–4074. [Google Scholar]; (b) Long JA; Harris NJ; Lammertsma K Formaldehyde Oxime ⇌ Nitrosomethane Tautomerism. J. Org. Chem 2001, 66, 6762–6767. [DOI] [PubMed] [Google Scholar]
- 24.Indeed, when formaldoxime trimer was used instead of nitromethane under otherwise optimal conditions, no methylamination product 4 is formed. See SI.
- 25.(a) Klunk WE; Engler H; Nordberg A; Wang Y; Blomqvist G; Holt DP; Bergström M; Savitcheva I; Huang GF; Estrada S; Ausen B; Debnath ML; Barletta J; Price JC; Sandell J; Lopresti BJ; Wall A; Koivisto P; Antoni G; Mathis CA; Langstrom B Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann. Neurol 2004, 55, 306–319. [DOI] [PubMed] [Google Scholar]; (b) Rowe CC; Ellis KA; Rimajova M; Bourgeat P; Pike KE; Jones G; Fripp J; Tochon-Danguy H; Morandeau L; O’Keefe G; Price R; Raniga P; Robins P; Acosta O; Lenzo N; Szoeke C; Salvado O; Head R; Martins R; Masters C; Ames D; Villemagne VL Amyloid Imaging Results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study of Aging. Neurobiol. Aging 2010, 31, 1275–1283. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Jones GC; Muñoz MP Isotopic Labelling in the Study of Organic and Organometallic Mechanism and Structure: An Account. J. Label. Compd. Radiopharm 2007, 50, 1072–1087. [Google Scholar]
- 27.(a) Mutlib AE Application of Stable Isotope-Labeled Compounds in Metabolism and in Metabolism-Mediated Toxicity Studies. Chem. Res. Toxicol 2008, 21, 1672–1689. [DOI] [PubMed] [Google Scholar]; (b) Chokkathukalam A; Kim DH; Barrett MP; Breitling R; Creek DJ Stable Isotope-Labeling Studies in Metabolomics: New Insights into Structure and Dynamics of Metabolic Networks. Bioanalysis 2014, 6, 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rinkel J; Dickschat JS Recent Highlights in Biosynthesis Research Using Stable Isotopes. Beilstein J. Org. Chem 2015, 11, 2493–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pons G; Rey E Stable Isotopes Labeling of Drugs in Pediatric Clinical Pharmacology. Pediatrics 1999, 104, 633–639. [PubMed] [Google Scholar]; (e) Kerfah R; Plevin MJ; Sounier R; Gans P; Boisbouvier J Methyl-Specific Isotopic Labeling: A Molecular Tool Box for Solution NMR Studies of Large Proteins. Curr. Opin. Struct. Biol 2015, 32, 113–122. [DOI] [PubMed] [Google Scholar]; (f) Gant TG Using Deuterium in Drug Discovery: Leaving the Label in the Drug. J. Med. Chem 2014, 57, 3595–3611. [DOI] [PubMed] [Google Scholar]
- 28.(a) Lennox AJJ; Lloyd-Jones GC Selection of Boron Reagents for Suzuki-Miyaura Coupling. Chem. Soc. Rev 2014, 43, 412–443. [DOI] [PubMed] [Google Scholar]; (b) Cox PA; Leach AG; Campbell AD; Lloyd-Jones GC Protodeboronation of Heteroaromatic, Vinyl, and Cyclopropyl Boronic Acids: PH-Rate Profiles, Autocatalysis, and Disproportionation. J. Am. Chem. Soc 2016, 138, 9145–9157. [DOI] [PubMed] [Google Scholar]; (c) Chen L; Sanchez DR; Zhang B; Carrow BP “cationic” Suzuki-Miyaura Coupling with Acutely Base-Sensitive Boronic Acids. J. Am. Chem. Soc 2017, 139, 12418–12421. [DOI] [PubMed] [Google Scholar]
- 29.A competition reaction involving reductive coupling of nitromethane under the standard condition in the presence of both 4-fluorophenylboronic acid and 4-chlorophenylboronic acid pinacol ester showed that boronic acid is more reactive than boronic acid pinacol ester (Page S37). Evidently, there is no inherent preference of the nitroalkane for the boronic ester.
- 30.(a) Itil TM; Akpinar S; Fink M Controlled clinical and quantitative EEG studies of triflubazam (ORF 8063) in patients with anxiety syndrome. Curr. Ther. Res 1976; 19, 307. [PubMed] [Google Scholar]; (b) Csanalosi I; Pereira-Oran J; Case G; Werblowsky J; Rickels K Triflubazam (ORF 8063), a new benzodiazepine in anxiety neurosis. Curr. Ther.Res 1977, 22, 166. [Google Scholar]; (c) Nicholson AN; Stone BM; Clarke CH Effect of the 1,5-benzodiazepines, clobazam and triflubazam, on sleep in man. Br. J. Clin. Pharmacol 1977, 4, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenstra DC; Rutjes FPJT; Mecinović J Triphenylphosphine-Catalysed Amide Bond Formation Between Carboxylic Acids and Amines. Chem. Commun 2014, 50, 5763–5766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


