Figure 4:
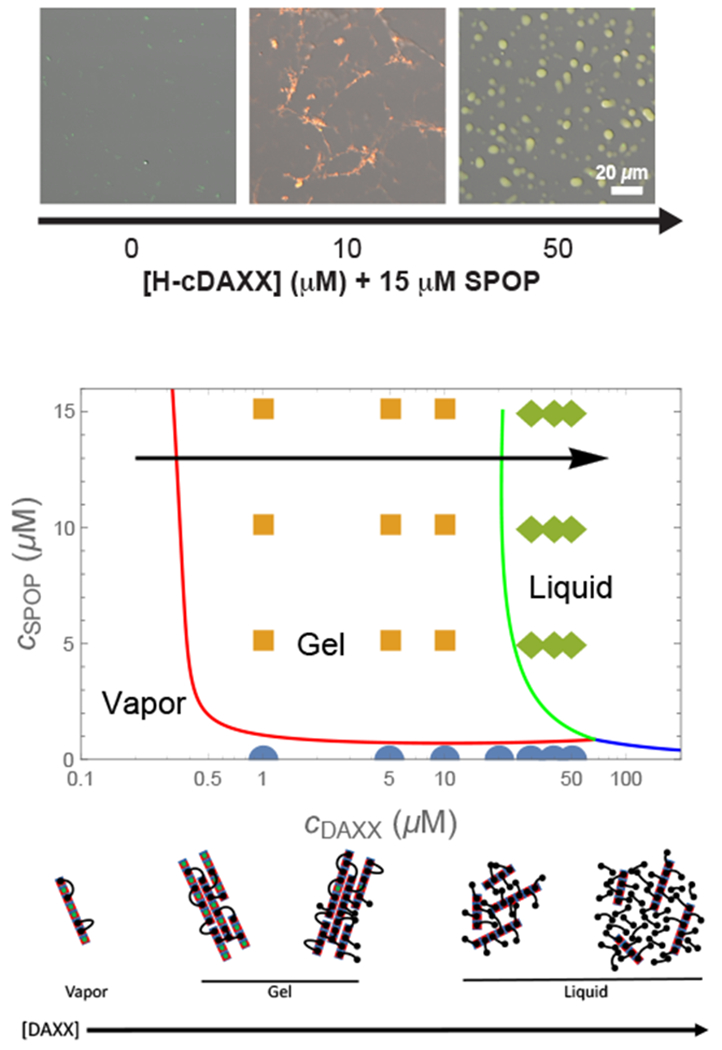
(top) Micrographs (fluorescence overlaid with DIG) of 15 μM SPOP solutions with increasing concentrations of cDAXX showing the vapor, gel, and liquid phases in 4% ficoll. (middle) Phase diagram calculated from Eq. 25, Eq. 26, and Eq. 28. Circles, squares, and diamonds indicate vapor, gel, and liquid phases observed experimentally.23 The calculation shows the behavior of SPOP assemblies and does not account for the pure DAXX fluid, (bottom) Cartoon of the progression of structures along the arrow in the phase diagram. At low DAXX concentration there are insufficient crosslinks to drive condensation and the SPOP assemblies are in the vapor phase. The gel is formed when the excess entropy of arranging the DAXX molecules is enough to offset the translational entropy of condensation. When the binding sites become saturated, double bound DAXX molecules become rare, however, the SPOP assemblies are still held together in the liquid phase by weak DAXX-DAXX interactions. Finally, at very high concentrations, DAXX monomers condense into a liquid that dissolves the SPOP assemblies.
