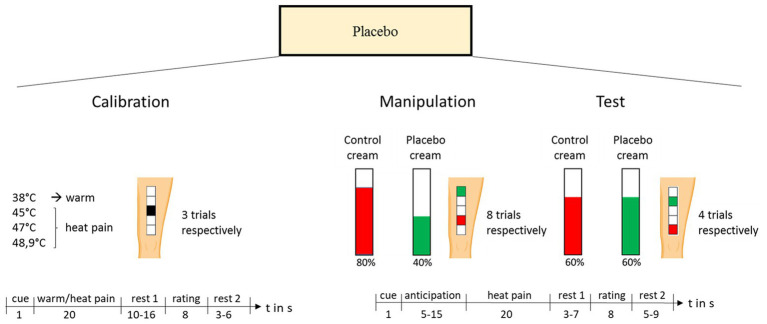
Figure 3.
Placebo paradigm. The placebo paradigm consisted of three phases: calibration, manipulation, and test. Before the experiment started, five 4 × 4 cm2 squares were drawn on the participants’ left thigh to mark the stimulation sites. Subjects were informed that they would receive pain-inhibition cream/inactive control cream on the skin areas outlined in green/red. The two upper/lower squares were outlined in green/red and designated as the site for later placebo/control cream stimulation. The assignment of placebo cream or control cream to the upper/lower patches was randomized across subjects. During the calibration phase, the middle square was used to determine the individual temperatures to evoke pain levels of 40, 60, and 80 on the VAS, ranging from 0 = no pain to 100 = unbearable pain. Therefore, a pseudo-random sequence of 12 20-s thermal stimuli with different intensities (38, 45, 47, and 48.9°C, three trials, respectively) was applied while the participants were asked to rate the intensity of each stimulus on the presented VAS. The individual temperatures evoking VAS ratings of 40, 60, and 80 were calculated via linear regression of the calibration ratings. Before the manipulation phase started, participants were told that they would be stimulated with the same pain level on both skin patches (placebo cream and control cream). Unbeknown to them, they were stimulated with a pain level of VAS 80 to the control site and with a pain level of VAS 40 for stimuli applied to the placebo site. The conditioning phase consisted of a pseudo-random sequence of placebo cream/control cream stimulation with eight trials each. The test phase consisted of a pseudo-random sequence with four trials per stimulation site. Importantly, participants were stimulated with the same temperature (equivalent to 60 on the VAS) at both stimulation sites (placebo-control). This physically identical stimulation allowed for the assessment of the individual placebo analgesic effect (i.e., reduced pain ratings under placebo cream compared with control cream).