Figure 4.
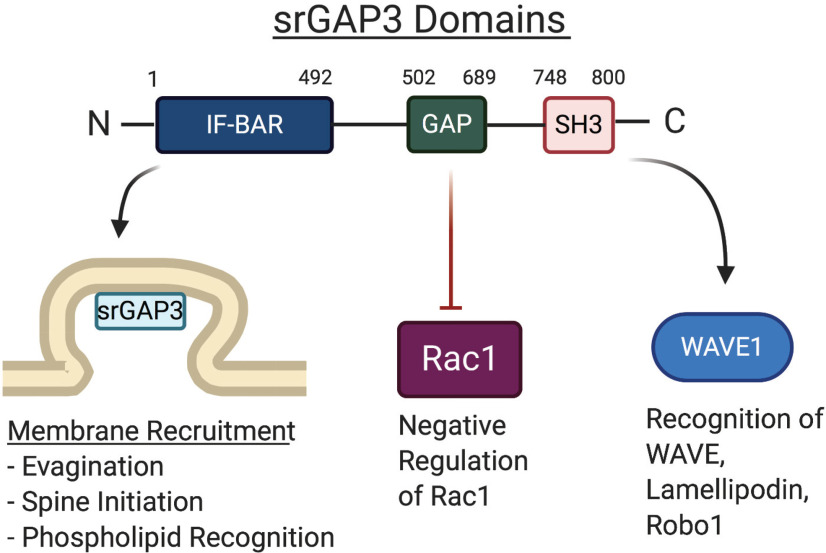
Functional domains of srGAP3 drive diverse interactions. The structure of srGAP3 is highly conserved with other srGAP proteins and contains three unique domains. At the N-terminal region is an IF-BAR domain that is composed of α-helical coils that bind the membrane as homodimers through recognition of specific membrane phospholipids, such as PIP2 and PIP3. In the central region, there is a GAP domain, which is specific for and inhibits the activity of Rac1. Toward the C-terminal tail of the protein is an SH3 domain that acts as a scaffolding adaptor for recognition of several different interactors, such as WAVE1, lamellopodin, and its canonical receptor Robo1. These regions allow srGAP3 to mediate a variety of different intracellular functions through interactions with a diverse set of interaction partners. The numbers depict the specific amino acid residues where each domain begins and ends in the human srGAP3 protein.