Figure 1.
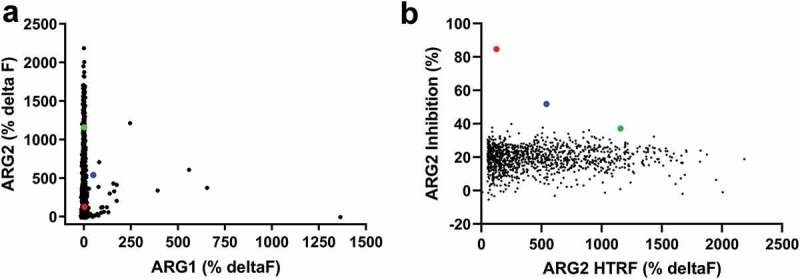
High throughput screening of ARG2 phage display round two outputs identified large numbers of human ARG2-specific binders, but few ARG2 inhibitors. Representative results illustrating that (a) clones sampled from ARG2 phage display selection outputs are highly specific for binding to ARG2 and not its paralogue, ARG1. (n = 1593/4400; ARG2 deltaF >100%, ARG1 deltaF <100%). In contrast, selection outputs contained (b) significantly fewer clones with an ability to inhibit recombinant human ARG2 (n = 1235). Lead candidate clones are highlighted: C0020185 ( ), C0020186 (
), C0020186 ( ) and C0020187(
) and C0020187( ). Figure 1(a,b) represent combined screening data from at least two independent experiments. Direct binding of scFv to biotinylated recombinant human trimeric ARG1 or ARG2 was measured using a HTRF® assay. ARG2 enzymatic activity measured in vitro by coupling ARG2-specific production of urea to colorimetric change. Each clone tested as a single data point. An irrelevant control scFv was included with each HTRF® and enzyme inhibition assay as a measure of nonspecific binding and nonspecific ARG2 inhibition, respectively (CEA6; mean percentage deltaF: 0.3 ± 1.9 and mean percentage ARG2 inhibition: 21.0 ± 3.9). The mean HTRF® percentage deltaF value (± standard deviation) for ‘maximum binding signal’ control wells (i.e. ARG2 incubated with an anti-ARG2 scFv, C0020100) was: % deltaF 386 ± 11. The mean percentage ARG2 inhibition value (± standard deviation) for ‘maximum ARG2 inhibition’ control wells (i.e., ARG2 incubated with CEA6 scFv but no L-Arginine) was: 0.39% ± 0.03%. All test and control scFv screened as unpurified periplasmic preparations.
). Figure 1(a,b) represent combined screening data from at least two independent experiments. Direct binding of scFv to biotinylated recombinant human trimeric ARG1 or ARG2 was measured using a HTRF® assay. ARG2 enzymatic activity measured in vitro by coupling ARG2-specific production of urea to colorimetric change. Each clone tested as a single data point. An irrelevant control scFv was included with each HTRF® and enzyme inhibition assay as a measure of nonspecific binding and nonspecific ARG2 inhibition, respectively (CEA6; mean percentage deltaF: 0.3 ± 1.9 and mean percentage ARG2 inhibition: 21.0 ± 3.9). The mean HTRF® percentage deltaF value (± standard deviation) for ‘maximum binding signal’ control wells (i.e. ARG2 incubated with an anti-ARG2 scFv, C0020100) was: % deltaF 386 ± 11. The mean percentage ARG2 inhibition value (± standard deviation) for ‘maximum ARG2 inhibition’ control wells (i.e., ARG2 incubated with CEA6 scFv but no L-Arginine) was: 0.39% ± 0.03%. All test and control scFv screened as unpurified periplasmic preparations.
