ABSTRACT
Antibodies that target a clinically relevant group of receptors within the tumor necrosis factor receptor superfamily (TNFRSF), including CD40 and CD95 (Fas/Apo-1), also require binding to Fc gamma receptors (FcγRs) to elicit a strong agonistic activity. This FcγR dependency largely relies on the mere cellular anchoring through the antibody’s Fc domain and does not involve the engagement of FcγR signaling. The aim of this study was to elicit agonistic activity from αCD40 and αCD95 antibodies in a myeloma cell anchoring-controlled FcγR-independent manner. For this purpose, various antibody variants (IgG1, IgG1N297A, Fab2) against the TNFRSF members CD40 and CD95 were genetically fused to a single-chain-encoded B-cell activating factor (scBaff) trimer as a C-terminal myeloma-specific anchoring domain substituting for Fc domain-mediated FcγR binding. The antibody-scBaff fusion proteins were evaluated in binding studies and functional assays using tumor cell lines expressing one or more of the three receptors of Baff: BaffR, transmembrane activator and CAML interactor (TACI) and B-cell maturation antigen (BCMA). Cellular binding studies showed that the binding properties of the different domains within the fusion proteins remained fully intact in the antibody-scBaff fusion proteins. In co-culture assays of CD40- and CD95-responsive cells with BaffR, BCMA or TACI expressing anchoring cells, the antibody fusion proteins displayed strong agonism while only minor receptor stimulation was observed in co-cultures with cells without expression of Baff-interacting receptors. Thus, our CD40 and CD95 antibody fusion proteins display myeloma cell-dependent activity and promise reduced systemic side effects compared to conventional CD40 and CD95 agonists.
KEYWORDS: Antibody fusion protein, bifunctional, CD40, CD95, TNFRSF
Introduction
With the approval of immune checkpoint inhibitors, immunotherapy has broadly entered clinical practice in the treatment of tumor diseases. In addition to the abrogation/neutralization of protumoral-acting immunosuppressive mechanisms by checkpoint inhibitors, the activation of immune cells, in particular T cells, is a major aim of immunotherapeutic approaches. Immune cell stimulatory approaches typically target T-cell activity either directly or indirectly. In the case of direct T cell receptor (TCR) activation, e.g., by use of bispecific T-cell engagers and chimeric antigen receptor (CAR) T-cells, the stimulatory effect is tumor-specific or at least highly tumor-associated. Tumor specificity is here of overwhelming importance to avoid severe or even deadly off-tumor effects. There are also immunotherapeutic attempts that aim to improve anti-tumor T-cell activity indirectly, by engagement of T-cell co-stimulatory receptors or dendritic cell (DC)-activating receptors of the tumor necrosis factor (TNF) receptor superfamily (TNFRSF).1–4 Additionally, attempts have been made to use the induction of apoptosis by TNFRSF receptors (TNFRs) for tumor therapy.5,6 The goal here is to directly exploit the increased sensitivity of some tumors for apoptosis, or to benefit from enhanced tumor cell apoptosis to increase cross-presentation of tumor antigens, and thus antitumoral immune response.
Multiple myeloma (MM) is a malignancy of differentiated B-cells, which localize to the bone marrow and cause osteolysis, bone pain and impaired hematopoiesis. Treatment of MM includes chemotherapy and stem cell transplantation, but also immunotherapy with daratumumab and elotuzumab, which are antibodies that enhance alloreactive natural killer cell cytotoxicity against CD38- or SLAMF7-expressing MM cells, respectively.7 Moreover, there are promising results from clinical trials with CAR-T cells and antibody-drug conjugates (ADCs) targeting B-cell maturation antigen (BCMA), which seems to be more selectively expressed and expressed at higher levels on MM cells than alternative MM targets such as CD38 and SLAMF7.8 Furthermore, published studies with lucatumumab and dacetuzumab, two antibodies targeting the DC-stimulatory TNFR CD40, revealed manageable adverse effects and modest clinical activity in Phase 1 studies with MM patients.9,10
Besides five decoy receptors, the TNFRSF comprises 24 different signaling-competent TNFR types in humans.11 Receptors of the TNFRSF interact with ligands of the TNFSF. The latter are typically expressed as trimeric transmembrane proteins, but also occur, after proteolytic processing, as soluble likewise trimeric molecules. The signaling-competent TNFRs can be assigned to one of two categories depending on their response to soluble TNFL trimers and anti-TNFR (αTNFR) antibodies.12,13 TNFRs of the first category, e.g., TNFR1 or lymphotoxin beta receptor, bind soluble ligand trimers and are fully activated this way. Similarly, bivalent IgG antibodies are usually sufficient to activate category I TNFRs. In contrast, TNFRs of the second category bind soluble ligand trimers, but are only limitedly, or not at all, activated thereby. Likewise, category II TNFRs are not or only poorly stimulated by receptor-specific IgG antibodies. Category II TNFRs include the T-cell costimulatory receptors 4–1BB, CD27 and OX40, as well as the DC-stimulatory CD40 receptor and the death receptors CD95 (Fas/Apo-1), TRAILR1 and TRAILR2.12,13
Research in the past decade revealed that antibodies specific for category II TNFRs regularly acquire strong agonism after FcγR binding, even if they otherwise block ligand binding and act as antagonists.12,13 In view of the overwhelming translational potential of the category II TNFRs, it is important to realize that FcγR binding of anti-category II TNFR antibodies, which is required to make them strongly agonistic, comes with severe limitations. First, optimal agonistic activity is limited in vivo due to the poor availability of FcγR-expressing cells and/or low cellular FcγR expression levels. Second, FcγR-mediated activities triggered by the antibody-FcγR interaction can counteract the therapeutic effects. Third, large antibody doses are typically required to overcome competition with serum IgGs for FcγR binding. Last, but not least, there can be dose-limiting side effects caused by the systemic activation of the targeted TNFR type (e.g., CD40: cytokine release/storm; CD95: hepatotoxicity).14–16 Using antibody fusion proteins with an anchoring domain (AD) enabling FcγR-independent binding to a cell surface-exposed anchoring target (AT), we could show that it is the sheer cell surface attachment that constitutes the agonism of anti-category II TNFR antibodies.12
Here, we used this principle finding to construct antibody fusion proteins displaying strong CD40 and CD95 agonism upon myeloma cell binding instead of FcγR binding. Thus, we demonstrate that derivatives of αCD40 and αCD95 antibodies devoid of FcγR-binding and harboring a scBaff anchoring domains show strong CD40- and CD95-activation in the presence of Baff receptor-expressing myeloma cells. This novel type of antibody fusion protein not only has the potential to overcome limitations that arise from FcγR engagement but also promises to reduce off-tumor activity of the targeted TNFRs.
Results
Fusion proteins of αCD40 and αCD95 with scBaff bind with high affinity to Baff-interacting receptors
IgG antibody-mediated activation of CD95 or CD40 typically requires IgG cross-linking or antibody binding to FcγRs.12,17-21 In accordance with the idea that it is the sheer cell surface anchoring that confers agonistic activity to FcγR-bound αCD40 and αCD95 antibodies, we recently demonstrated that αCD40 and αCD95 fusion proteins harboring a CD20-specific scFv domain at the C-terminus of the heavy chain acquire strong agonism upon binding to CD20-expressing cells.12 Therefore, we wondered whether αCD40 and αCD95 fusion proteins with an MM-specific anchoring domain allow in a similar fashion FcγR-independent MM cell-mediated activation of CD40 and CD95. To investigate this question, we genetically fused a single-chain encoded trimer of soluble Baff protomers (scBaff) to the C-terminus of the heavy chain of IgG1N297A and Fab2 variants of the αCD40 antibody G28.5 and the αCD95 antibody E0922,23 resulting in the antibody fusion proteins αCD40N297A-scBaff, αCD95N297A-scBaff, αCD40Fab2-scBaff and αCD95Fab2-scBaff (Figure 1). Baff is a ligand of the TNFSF and interacts with three receptors of the TNFRSF: TACI, BCMA and Baff receptor (BaffR).24 Expression of all three Baff-interacting receptors is restricted to cells of the B-cell compartment, and especially BCMA is highly expressed on plasma cells and myeloma cells.24,25 Indeed, BCMA was successfully targeted in clinical trials with ADCs, CAR-T cells and bispecific T-cell engagers.25
Figure 1.
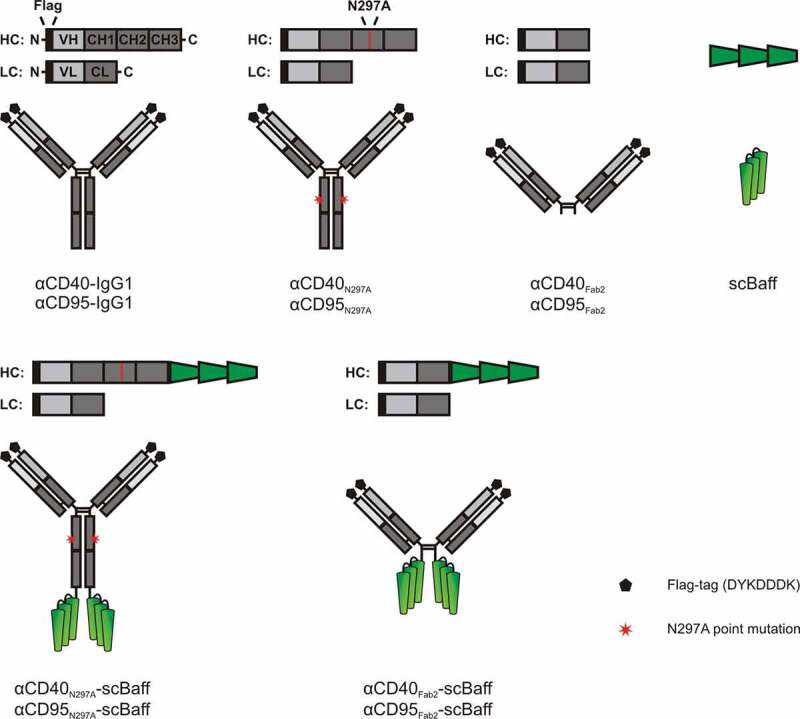
Domain architecture of the antibodies and antibody fusion proteins investigated in this study.
Cellular binding studies with transiently TACI-, BCMA- and BaffR-expressing transfectants (supplemental data Fig. S1) and Gaussia princeps luciferase (GpL)-modified variants of soluble Baff and the four antibody-scBaff fusion proteins revealed comparable affinities for the three types of Baff receptors in the range of 0.5 to 2.6 nM (Figure 2a, Table 1). This indicates that the antibody parts of the antibody-scBaff fusion proteins have no major effect on the interaction of the scBaff domain and the Baff receptors. Vice versa, there was binding of the GpL-tagged αCD40- and αCD95-scBaff fusion proteins with nM affinity to CD40- and CD95-expressing transfectants (Figure 2b, Table 1).
Figure 2.
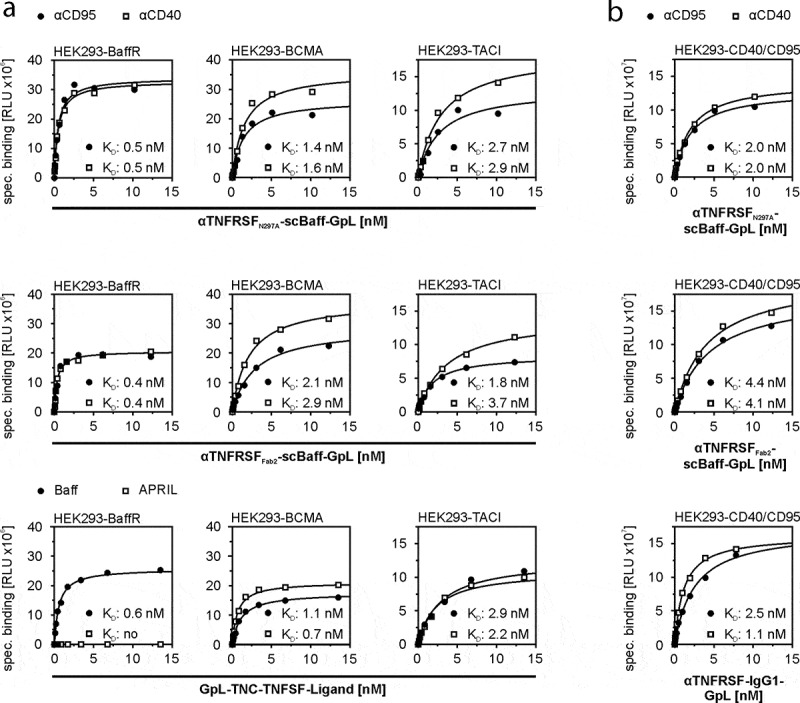
Equilibrium binding of GpL-tagged aCD40- and αCD95-scBaff fusion proteins to CD40 and CD95 and the Baff-interacting receptors BaffR, TACI and BCMA. (a,b) HEK293 transfectants transiently expressing the Baff-interacting receptors (a) or CD40 or CD95 (b) were used to determine total binding. Empty vector (EV) transfected cells were used to determine unspecific binding. Specific binding was calculated by subtraction of the unspecific binding values from the corresponding total binding values. Binding of GpL fusion proteins of TNC-Baff and TNC-APRIL was analyzed for comparison. Data of one representative experiment for each of the investigated interactions are shown. Averaged KD-values of 4 independent experiments are listed in Table 1.
Table 1.
Affinity of GpL-tagged TNC-Baff and CD40- and CD95-specific antibody-scBaff fusion proteins for cell-expressed receptors.
| Construct | KD values (nM) of independent experiments | Averaged KD (nM) |
|---|---|---|
| BaffR | ||
| TNC-Baff | 0.6, 0.6, 0.5, 0.5 | 0.5 ± 0.1 |
| αCD40N297A-scBaff | 0.5, 0.4, 0.2, 1.3 | 0.6 ± 0.5 |
| αCD40Fab2-scBaff | 0.4, 0.7, 0.4, 1.3 | 0.7 ± 0.4 |
| αCD95N297A-scBaff | 0.5, 0.5, 0.2, 1.2 | 0.6 ± 0.4 |
| αCD95Fab2-scBaff | 0.4, 0.6, 0.3, 0.8 | 0.5 ± 0.2 |
| BCMA | ||
| TNC-Baff | 2.2, 4.0, 1.1, 2.3 | 2.4 ± 1.2 |
| αCD40N297A-scBaff | 1.6, 2.5, 3.7, 3.6 | 2.8 ± 1.0 |
| αCD40Fab2-scBaff | 1.9, 2.9, 2.8, 2.1 | 2.4 ± 0.5 |
| αCD95N297A-scBaff | 1.4, 3.6, 4.3, 1.8 | 2.8 ± 1.4 |
| αCD95Fab2-scBaff | 1.5, 2.1, 2.6, 1.6 | 1.9 ± 0.5 |
| TACI | ||
| TNC-Baff | 2.9, 3.0, 2.2, 2.1 | 2.6 ± 0.5 |
| αCD40N297A-scBaff | 1.5, 2.0, 2.8, 2.9 | 2.3 ± 0.7 |
| αCD40Fab2-scBaff | 3.3, 1.6, 3.7, 2.0 | 2.6 ± 1.0 |
| αCD95N297A-scBaff | 1.5, 1.7, 3.0, 2.7 | 2.2 ± 0.7 |
| αCD95Fab2-scBaff | 2.0, 1.5, 1.8, 1.8 | 1.8 ± 0.2 |
| CD40 | ||
| αCD40-WT | 1.2, 1.3, 1.1, 1.0 | 1.1 ± 0.1 |
| αCD40N297A-scBaff | 1.4, 1.2, 1.7, 2.0 | 1.6 ± 0.3 |
| αCD40Fab2-scBaff | 1.8, 2.5, 4.1, 2.6 | 2.7 ± 1.0 |
| CD95 | ||
| αCD95-WT | 1.7, 1.9, 2.5, 3.8 | 2.5 ± 0.9 |
| αCD95N297A-scBaff | 1.7, 2.1, 3.0, 2.0 | 2.2 ± 0.5 |
| αCD95Fab2-scBaff | 4.6, 4.3, 4.9, 4.4 | 4.5 ± 0.3 |
Binding to Fcγ receptors or Baff-interacting receptors unleashes the agonistic activity of αCD40 and αCD95 antibodies and antibody fusion proteins
Next, we analyzed the effect of the inhibitory Fcγ-receptor FcγRIIb (CD32B), which has a low affinity for human IgG1, of the stimulatory Fcγ receptor FcγRIa, which has a high affinity for human IgG1, and of BaffR on the receptor stimulatory activity of the antibodies αCD40-IgG1 and αCD95-IgG1 and the antibody fusion proteins αCD40N297A-scBaff, αCD95N297A-scBaff, αCD40Fab2-scBaff and αCD95Fab2-scBaff. The experiments were performed with supernatants containing the antibodies and antibody fusion proteins of interest. To assess CD40 activation by αCD40-IgG and the αCD40 fusion proteins, cell culture supernatants containing these reagents were added to HT1080-CD40 cells along with HEK293 cells transiently transfected with expression plasmids encoding FcγRIIb, FcγRIa and BaffR or, as a negative control, with empty vector (EV). Since HT1080-CD40 cells produce much higher amounts of interleukin-8 (IL8) than HEK293 cells in response to CD40 activation, production of IL8 was monitored the next day as an easily quantifiable indicator of CD40 activation.
Similarly, activation of CD95 by the αCD95 antibody and its derivatives was evaluated by adding them, along with the various HEK293 transfectants, to HT1080 cells. Cell death induction in the latter was then monitored the next day by crystal violet staining of the remaining plastic-adhered surviving cells. As observed before for the murine FcγIIB,12 FcγRIIb, but also FcγRIa, enabled αCD40-IgG1 and αCD95-IgG1 to trigger robust TNFRSF receptor (TNFR) signaling (Figure 3a,b). Similarly, FcγRIIb and FcγRIa enabled αCD40-IgG1-scBaff and αCD95-IgG1-scBaff to robustly stimulate CD40/CD95 signaling (Figure 3a,b). Not unexpectedly, the presence of BaffR-transfected cells had no effect on the ability of αCD40-IgG1 and αCD95-IgG1 to stimulate TNFR signaling (Figure 3a,b). In accordance with the fact that the N297A mutation strongly reduces the ability of IgG1 to bind FcγRs, there was no or only a minor enhancing effect of FcγRIIb- and FcγRIa-expressing cells on the ability of αCD40N297A-scBaff and αCD95N297A-scBaff to stimulate induction of IL8 and apoptosis (Figure 3a,b). In the presence of BaffR-expressing transfectants, however, both antibody fusion proteins acted as strong TNFR agonists (Figure 3a,b). Similarly, αCD40Fab2-scBaff and αCD95Fab2-scBaff, which lack a FcγR-interacting Fc domain, elicited strong CD40 and CD95 activation in cultures with BaffR-expressing cells, but showed only minor activity in the presence of FcγRIIb and FcγRIa transfectants (Figure 3a,b). Thus, with respect to conferring robust agonistic activity, scBaff domain-mediated anchoring to BaffR seems to be as efficient as Fc domain-mediated anchoring to FcγRs.
Figure 3.
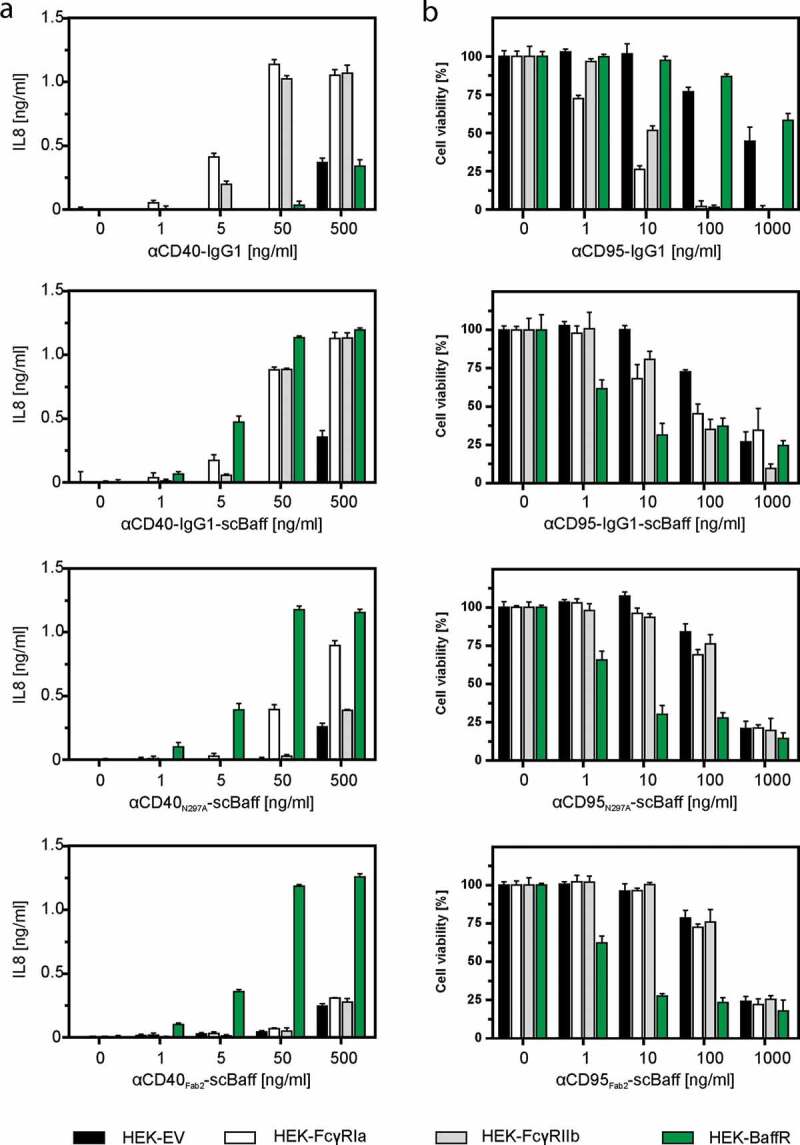
Effect of FcγR- and BaffR-binding on the agonistic activity of CD40- and CD95-specific antibodies and antibody-scBaff fusion proteins. (a,b) HT1080-CD40 (a) and HT1080 cells (b) were seeded in 96-well plates and were incubated the next day with HEK293 cells transiently transfected with empty vector (EV) or expression plasmids encoding the stimulatory Fcγ receptor FcγRIa, the inhibitory Fcγ-receptor FcγRIIb or BaffR along with increasing concentrations of the indicated CD40- (a) and CD95-specific (b) antibodies and antibody fusion proteins. In the case of the CD95-specific reagents 2.5 µg/ml CHX was added to sensitize HT1080 cells for apoptosis induction. The next day, CD40-mediated IL8 induction was quantified by ELISA (a) and CD95-induced apoptosis was quantified by crystal violet staining of the surviving adherent cells (b).
To verify that the properties of the antibody fusion proteins in the supernatants do not differ from the properties of the purified proteins, we exemplarily purified and reanalyzed αCD40N297A-scBaff and αCD40Fab2-scBaff. The fusion proteins were purified by αFlag affinity chromatography on αFlag mAb M2 agarose by the help of a N-terminal Flag tag contained in the antibody chains (Figure 4a,b). Similar to the αCD40-scBaff fusion protein-containing supernatants, purified αCD40N297A-scBaff and purified αCD40Fab2-scBaff induced with high-efficiency IL8 production in HT1080-CD40 cells in the presence of BaffR-transfected HEK293 cells (Figure 4c, supplemental data Fig. S2A). Not unexpectedly, BCMA- and TACI-expressing transfectants, but not EV-transfected control cells, also enabled purified αCD40N297A-scBaff and purified αCD40Fab2-scBaff to trigger strong IL8 production in HT1080-CD40 cells (Figure 4c, supplemental data Fig. S2A). Moreover, pretreatment of the BaffR-, BCMA- and TACI-expressing transfectants with a high concentration of soluble Baff strongly reduced IL8-induction by the purified αCD40-fusion proteins (Figure 4d, supplemental data Fig. S2B). This confirmed the hypothesis that binding of the antibody-scBaff fusion proteins via their scBaff domain to BaffR, BCMA and TACI was the crucial factor for the strongly enhanced CD40-stimulatory activity of these reagents. Thus, purification did not affect the FcγR-independent agonistic activity of BaffR-, TACI- and BaffR-bound αCD40N297A-scBaff and αCD40Fab2-scBaff.
Figure 4.
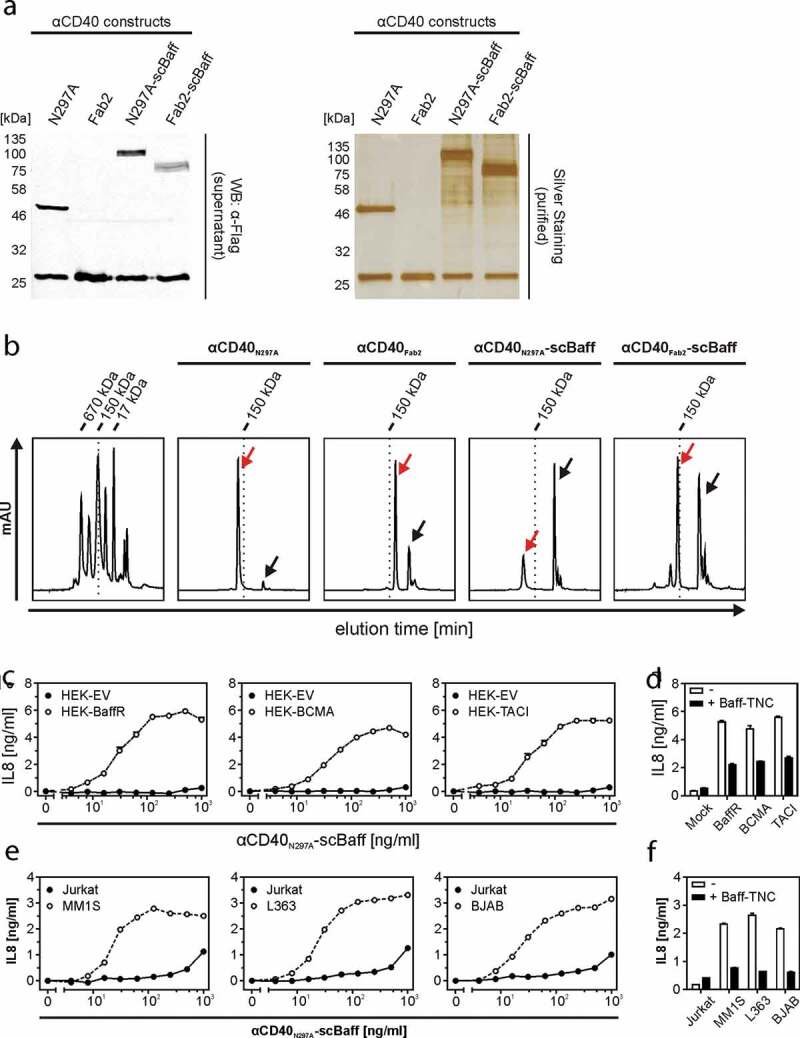
CD40 stimulation by purified αCD40N297A-scBaff. (a) The indicated proteins were purified by affinity purification on anti-Flag agarose and were analyzed by SDS-PAGE and silver staining. (b) Gel filtration analysis of the various purified αCD40 variants. The low molecular weight peaks marked by the black arrows indicate the position of the Flag peptide used for elution of the Flag-tagged antibodies. The red arrows indicate the peak of the dimeric IgG1/Fab2 fusion proteins. The left panel shows the analysis of a mixture of protein standards of known size. The positions of the 17, 150 and 670 kDa marker proteins are indicated. (c) HT1080-CD40 were seeded in 96-well plates and were challenged the next day with HEK293 cells transiently transfected with empty vector (EV) or expression plasmids encoding the indicated receptors along with increasing concentrations of αCD40N297A-scBaff. Next day, CD40 activation was evaluated by analysis of IL8 production by ELISA. (d) Co-cultures as described in “C” were pretreated for 30 min with or without 5 µg/ml Flag-TNC-Baff and were then stimulated with 200 ng/ml of αCD40N297A-scBaff before IL8 production was evaluated the next day by ELISA. (e) U2OS cells seeded in 96-well plates were challenged the next day with 4 × 104 MM.1S, L363, BJAB or Jurkat cells along with the indicated concentrations of αCD40N297A-scBaff. One day later, CD40 activation was again evaluated by assaying IL8 production by ELISA. (f) Cells were again seeded in 96-well plates and were supplemented the next day as indicated with 4 × 104 MM.1S, L363, BJAB or Jurkat cells. Co-cultures were pretreated for 30 min with or without 5 µg/ml Baff-TNC, were then stimulated with 200 ng/ml of the CD40-specific antibody-scBaff fusion proteins and the next day CD40 activation was again assessed by means of IL8 ELISA.
To verify that endogenous expression levels of BaffR, BCMA and TACI and of CD40 and CD95 are also sufficient to ensure efficient agonism of αCD40N297A-scBaff, αCD95N297A-scBaff, αCD40Fab2-scBaff and αCD95Fab2-scBaff, we also performed coculture experiments with cells expressing the relevant molecules endogenously. For this purpose, we used U2OS cells, which express CD40 endogenously, as CD40 responder cells producing robust amounts of IL8 in response to CD40 stimulation but lack expression of Baff receptors (supplemental data Figs. S3 and S4). HT1080 cells expressing endogenous CD95 were furthermore used as CD95 responder cells. The MM cell lines MM.1S and L363 expressing BCMA and TACI and the B-cell lymphoma cell line BJAB, which primarily express BaffR, were furthermore used as anchoring cells that do not produce IL8 in response to TNFR activation (supplemental data Figs. S3 and S4). As a negative control Jurkat cells, which express none of the three Baff-interacting receptors and display no IL8 induction, were used. In the presence of Jurkat cells, the purified αCD40-scBaff fusion proteins showed only a very minor stimulatory effect on U2OS cells at concentrations >500 ng/ml (Figure 4e, supplemental data Fig. S2C). In the presence of the cell lines that express one or two of the Baff-interacting receptors, however, the αCD40 fusion proteins triggered strong IL8 production in U2OS cells already at low concentrations of 10–25 ng/ml (Figure 4e, supplemental data Fig. S2C). CD40-mediated IL8 induction triggered by the αCD40scBaff fusion proteins was again inhibited in cocultures supplemented with soluble Baff (Figure 4f, supplemental data Fig. S2D).
The Baff receptor anchoring-dependent mode of action also overcomes the inhibitory effect that irrelevant IgG species have on anti-TNFR antibodies with FcγR-dependent agonistic activity. Thus, high but physiological relevant concentrations of an irrelevant IgG1 strongly inhibited IL8 induction by FcγR-anchored αCD40-IgG1-scBaff, but showed no effect on the ability of BaffR-anchored αCD40-IgG1-scBaff to stimulate IL8 production (supplemental data Fig. S5).
The αCD95-scBaff fusion proteins displayed a similar behavior as the αCD40-scBaff fusion proteins. The αCD95-scBaff fusion proteins triggered cell death in H1080 cells only at higher concentrations (>100 ng/ml) in the presence of Jurkat cells, but in the presence of BJAB and L363 cells the same constructs triggered cell death at ~100-fold lower concentrations (Figure 5a). Cell death-induction by αCD95N297A-scBaff and αCD95Fab2-scBaff was inhibited in cocultures supplemented with soluble Baff (Figure 5b). CD95 also has the ability to trigger IL8 production, especially when apoptosis induction is prevented by caspase inhibition. Accordingly, in the presence of the caspase inhibitor ZVAD, αCD95N297A-scBaff and αCD95Fab2-scBaff induced IL8 production in HT1080 cells cocultured with BJAB, MM.1S and L363 cells in a scBaff-dependent fashion (Figure 5c,d). Again, high concentrations of an irrelevant IgG1 showed no effect on the scBaff-mediated αCD95 agonism, but inhibited FcγR anchoring-dependent CD95 activation by αCD95-IgG1-scBaff (supplemental data Fig. S5). Baff receptor expressing cell lines typically also express CD95 and some of them are sensitive for CD95-mediated cell death. Accordingly, we found that BJAB cells alone are killed by low concentrations of αCD95N297A-scBaff and were partly rescued by cotreatment with a soluble Baff construct as competitor for BaffR binding (supplemental data Fig. S6).
Figure 5.
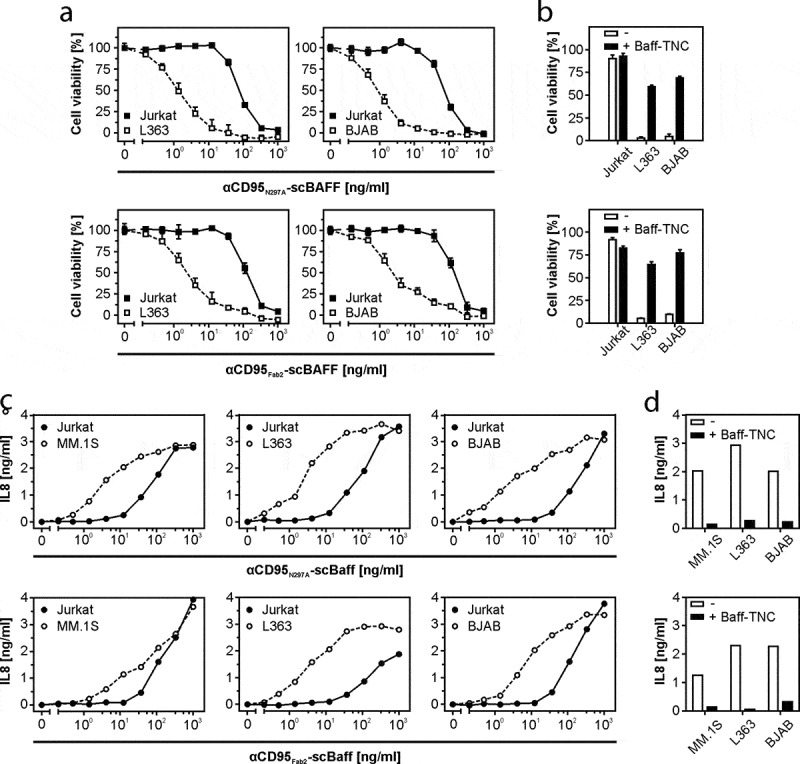
Lymphoma- and myeloma cell-restricted activation of CD95 by antibody-scBaff fusion proteins. (a) HT1080 were cultivated in 96-well plates and were challenged in the presence of 2.5 µg/ml CHX with 4 × 104 L363, BJAB or Jurkat cells along with αCD95N297A-scBaff and αCD95Fab2-scBaff. Next day, remaining viable plastic adhered cells were quantified by crystal violet staining. (b) HT1080 cell were again seeded in 96-well plates and were supplemented the next day as indicated with 4 × 104 L363, BJAB or Jurkat cells. Co-cultures were pretreated for 30 min with or without 5 µg/ml Baff-TNC and were then stimulated with 20 ng/ml of the αCD95-antibody scBaff fusion proteins, respectively. On the next day, CD95 activation was evaluated by determination of cellular viability. (c) Cocultures of HT1080 with 4 × 104 MM.1S, L363, BJAB or Jurkat cells were cultivated in 96-well plates and were challenged in the presence of 2.5 µg/ml CHX and 20 µM zVAD along with αCD95N297A-scBaff and αCD95Fab2-scBaff. On the next day, NFκB signaling was assessed by means of IL8 ELISA. (d) Co-cultures were set up as in “C” and were pretreated for 30 min with or without 5 µg/ml Baff-TNC. Cells were then stimulated with 20 ng/ml of the αCD95-antibody scBaff fusion proteins and on the next day CD95-mediated IL8 production was again assayed by ELISA.
Discussion
Targeting CD40 and CD95 has been considered for the treatment for MM. In the case of CD40, two therapeutic goals are plausible: 1) Destruction of myeloma cells with the help of antibody-dependent cell-mediated cytotoxicity (ADCC)-inducing αCD40 antibodies, exploiting the fact that CD40 is expressed on many MM cells; and 2) agonistic αCD40 antibodies could be used to enhance the body’s immune response against MM by stimulating DC and other antigen-presenting cells. Several αCD40 antibodies have been tested in vivo in preclinical studies and showed good anti-myeloma activity.26–29 In most of these studies, ADCC was observed and claimed as the mode of action. Since antibodies against human CD40 had been tested, the experiments were performed in mice lacking T- and B-cells. Therefore, the studies have no relevance regarding the question to what extent FcγR-bound, and therefore agonistic, αCD40 antibodies favor a myeloma-specific immune response. Early clinical studies in MM patients with lucatumumab, a human αCD40 IgG1 antibody blocking the interaction with the CD40 ligand CD154 (CD40L), and dacetuzumab, a humanized αCD40 IgG1, which does not interfere with the CD154–CD40 interaction, revealed dose-limiting but manageable adverse events.9,10 However, there was also only modest clinical activity in these Phase 1 studies. Importantly, since FcγR-binding of these αCD40 antibodies not only results in triggering of ADCC but presumably also converts them into potent CD40 agonists, it is unclear to which extent these two mechanisms contributed to the dose-limiting adverse effects. In any case, it appears plausible that the adverse effects could be reduced by myeloma cell-restricted CD40 activation. αCD40 antibody-scBaff fusion proteins, as described here, promise such myeloma-associated CD40 activation. While the use of antibody variants (e.g., N297A-mutated IgG1 or Fab2) devoid of FcγR-binding ability prevents systemic effects, the scBaff anchoring domain ensures high-affinity binding to Baff receptors and strong CD40 activation on neighboring cells.
Future preclinical studies in vivo must now show whether Baff receptor-restricted myeloma cell-associated activation of CD40 has the power to mount a relevant immune response against myeloma. It is worth noting that binding of the αCD40-scBaff antibody fusion proteins to BCMA, BaffR or TACI can result in the activation of these receptors. In this respect, we observed that αCD40N297A-scBaff stimulates IKBα phosphorylation, a hallmark event in the classical NF-kappaB pathway, in MM.1S cells with an efficiency comparable to the strongly agonistic hexameric Baff and CD40L variants (supplemental data Fig. S7). Despite this activity, αCD40N297A-scBaff showed no major effects on MM.1S cell growth/proliferation at normal and low serum conditions (supplemental data Fig. S7). Stimulatory effects of Baff receptors on proliferation and cell survival in myeloma cells have been reported.8,25 Thus, future in vivo studies evaluating the therapeutic immunostimulatory activity of αCD40-scBaff antibody fusion proteins must also consider direct effects of αCD40N297A-scBaff on MM cells that may antagonize the anticipated immunotherapeutic activity.
As is the case for many tumors, myeloma cells are often sensitive to apoptosis induction by CD95. Accordingly, a number of studies have shown that the growth of cells in mice is strongly inhibited when these cells have been transfected with CD95L.30,31 In view of the prominent cell death-inducing activities of CD95 in vitro, it has been assumed that the reduced growth of CD95L transfected cells in vivo is primarily related to cell death induction. It was shown, however, that the growth inhibitory effects of CD95L in these models are less due to apoptosis induction, and more related to CD95-induced release of chemokines and cytokines and subsequent recruitment of immune cells.30,31 Thus, as in the case of CD40, a paracrine mode of action appears most relevant for the potential anti-myeloma activity of αCD95-scBaff antibody fusion proteins. Of course, this does not rule out that autocrine cell death induction might have an additional beneficial effect.
Expression of BCMA is very specific for terminally differentiated B cells and plasma cells.8,24 BaffR is expressed on most B cell populations and TACI expression has been reported for activated B cells.24 There is furthermore a contradictory data situation regarding TACI expression on T cells.24 All three receptor types, especially BCMA, are also expressed on MM cells.8,24,32-34 Since the B cells, especially differentiated B cells and plasma cells, are dispensable, BCMA, TACI and BaffR appear to be excellent targets for cytotoxic therapeutics. Indeed, high response rates have been reported in clinical trials with BCMA-targeted CAR-T cells and BCMA-specific ADCs applied to patients with relapsed and refractory MM.35–40
The anti-myeloma activity of BCMA-targeted CAR-T cells and ADCs, however, is often only temporary due in part to the loss of BCMA expression in response to therapy. The use of scBaff as an MM-targeting domain has the potential to reduce the efficacy of this escape mechanism. Since Baff not only interacts with BCMA but also with high affinity with TACI and BaffR,8,24 it is tempting to speculate that CD40- and CD95-specific antibodies with a scBaff anchoring domain have the potential to kill myeloma cells that had escaped from BCMA-targeted CAR-T cells or BCMA-specific ADCs. CAR-T cells only affect cells that express the CAR-recognized antigen, thus sparing antigen-negative cells. In contrast, αCD40 and αCD95 antibodies with a scBaff anchoring domain should also be able to enhance the immune response against a Baff receptor-negative subfraction of MM cells, due to DC stimulation or enhanced cross-presentation of tumor antigens released by apoptotic tumor cells. Therefore, although initially triggered by antigen-expressing cells, this response type also affects antigen-negative tumor cells. αCD40 and αCD95-antibody fusion proteins with Baff receptor-restricted activity thus have the potential to synergistically act with BCMA-specific CAR-T cells. In favor of this idea, it has been reported that BCMA-specific CAR-T cells that have been genetically engineered to express CD40L are superior to the corresponding conventional CAR-T cells and also affect BCMA-negative myeloma cell subpopulations.41,42
Only preliminary and incomplete conclusions can be drawn from the experience with BCMA-targeted CAR-T cells and BCMA-specific ADCs due to the paracrine mode of action of αCD40 and αCD95-scBaff fusion proteins. The side effects of BCMA-targeted CAR-T cells or anti-BCMA ADCs are likely related to the depletion of the B-cell compartment; however, αCD40 and αCD95-scBaff fusion proteins might elicit side effects due to stimulation of CD40 and CD95 on non-B cells in the neighborhood of B-cells. CD40- and CD95-expressing normal cells, such as fibroblasts, endothelial cells, epithelial cells and hepatocytes, have limited contact with B cells. Therefore, it is tempting to speculate that B cell associated mode of CD40/CD95 activation may result in fewer side effects compared with the use of intrinsically, and thus systemically, active agonists for these receptors (e.g., oligomeric ligand trimers). Notably, direct clinical targeting of CD95 with agonists cannot be considered because high liver toxicity has been reported for several systemically active CD95 agonists.15,16 The αCD95-antibody scBaff fusion proteins with B cell- and myeloma cell-dependent CD95 agonism (Figures 3, 5) potentially have strongly reduced liver toxicity compared to conventional CD95 agonists. Thus, the construct type described in our study could have the potential to make a new effector activity, CD95-induced cell death, available for myeloma treatment.
Materials and methods
Cell lines and reagents
BJAB, HEK293, HT1080, Jurkat, L363, MM.1S and U2OS cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ) (Braunschweig, Germany) or the American Type Culture Collection (ATCC) (Rockville, MD, USA). HT1080-CD40 cells have been described elsewhere.43 With the exception of U2OS cells, all cell lines were cultured in RPMI1640 medium (#R8758, Sigma-Aldrich, Munich, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (#10500064, Gibco, Schwerte, Germany). U2OS cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (#D5796, Sigma-Aldrich, Munich, Germany) supplemented with 10% heat-inactivated FBS. pCMV-SPORT6-based expression plasmids encoding FcγRIa, FcγRIIb and BaffR were obtained from SourceBioScience (Nottingham, UK). Expression plasmids encoding CD40, CD95, BCMA and TACI were a kind gift from Pascal Schneider (Lausanne, Switzerland). Production and characteristics of Fc-CD40L were described elsewhere.43
Molecular cloning, production and purification of ligands, antibodies and antibody fusion proteins
The light and heavy chain variants of the various antibodies and antibody fusion proteins were cloned into the pCR3 expression plasmid (Invitrogen). For cloning of the light and heavy chains, synthetic DNA fragments (Geneart, Thermo Fisher Scientific, Waltham, MA, USA) encoding the VH and VL domains of αCD40 and αCD95 were used; the fragments were designed according to the publicly accessible sequences of the human CD40-specific antibody G28.5 (acc. no. AJ853736) and the human CD95-specific antibody E09 (PDB entry 3TJE), respectively. We furthermore used DNA fragments encoding the constant part of the human IgG1 heavy (acc. no. AFR78282.1) and light chain (acc. no. AAD29610.1). All light and heavy chain-encoding expression plasmids comprise a N-terminal Flag-encoding sequence (DYKDDDDK) for simple purification and detection. The expression plasmids for the heavy chains of the various scBaff antibody fusion proteins encode for three copies of aa 137–285 of human BAFF (acc. no. Q9Y275.1), which were connected by glycine-serine linkers fused to the C-terminus of the heavy chain of the corresponding antibody. Expression plasmids for GpL-tagged light chains were obtained by fusing a GpL-encoding DNA fragment to the 3ʹ end of the light chain-encoding DNA fragment of the antibodies. The expression plasmids for TNC-Baff, GpL-TNC-BAFF and GpL-TNC-APRIL encoding soluble Baff/APRIL variants containing the small trimer stabilizing tenascin-C trimerization domain and partially a GpL domain have been described elsewhere.44 An expression plasmid encoding the hexameric Baff variant Fc-TNC-Baff was obtained by genetic fusion of the human IgG1 Fc domain to the N-terminus of TNC-Baff.
For the production of the ligand variants and the various antibodies and antibody fusion proteins, HEK293 cells were transiently transfected as described elsewhere.45 In brief, 12 µg plasmid DNA of either the ligand-encoding expression plasmids or 1:1 mixtures of the heavy and light chain-encoding plasmids of interest were pre-incubated with 36 µl of a 1 mg/ml solution of polyethylenimine (PEI) (#23966-1, Polysciences Europe GmbH, Eppelheim, Germany) in 2 ml serum-free RPMI1640 medium. Medium of HEK293 cells seeded the day before in 15 cm tissue culture plates were changed with medium to serum-free RPMI1640 medium supplemented with 1% penicillin/streptomycin and then supplemented with the plasmid/PEI mixture. The next day, medium was changed to RPMI1640 medium supplemented with 2% FBS and 1% penicillin/streptomycin. After an additional 5–6 d, cell culture supernatants were collected and cleared by centrifugation. The concentration of the produced proteins was determined by anti-Flag Western Blotting and comparison with a Flag-tagged standard protein or, where applicable, by measuring luciferase activity. Proteins were purified by affinity chromatography on anti-Flag agarose beads (#A2220, Sigma-Aldrich) according to the manufacturer’s recommendations.
SDS-PAGE, silver staining and gel filtration of purified proteins
To analyze purity, the various recombinant proteins were separated by SDS-PAGE. Samples and molecular weight markers of known concentrations (#17-0446-01, Amersham LMW calibration Kit, GE Healthcare, Chicago, IL, USA) were boiled in Laemmli sample buffer containing 5% (v/v) β-mercaptoethanol for 5 minutes at 95°C and subjected to SDS-PAGE on 12.5% (w/v) polyacrylamide gels. Gels were stained using the Pierce Silver Stain Kit (#24612, Thermo Fisher) according to the protocol of the supplier. Purified antibodies and antibody fusion proteins (50–200 µg) were further analyzed for their native weight and potential protein aggregation by gel filtration on a MabPac SEC-1 column (#088460, Thermo Fisher) using the UltiMate 3000 HPLC system (Thermo Fisher) and the aqueous SEC-1 column performance check standard (#AL0-3042, Phenomenex, Torrance, CA, USA).
Western blotting
Proteins separated by SDS-PAGE were wet electrotransferred to nitrocellulose membranes. The recombinant proteins were detected using the αFlag antibody M2 as primary antibody (#F1804, Sigma-Aldrich) and an IRDye 800 labeled anti-mouse IgG from goat as secondary antibody (#925-32210, LI-COR Biosciences, Lincoln, NE, USA) and subsequent detection of fluorescence with a LICOR Odyssey phosphorimager (LI-COR Biosciences).
Transient expression of receptors and flow cytometry
For binding studies, HEK293 cells were PEI-transfected with expression plasmids encoding the receptor of interest (BaffR, BCMA, TACI, FcγRIa, FcγRIIb) as described above for antibody production. One or two days after transfection, cells were harvested and 0.3–1.0 × 106 cells were washed with ice-cold phosphate-buffered saline (PBS) and incubated at 4°C with PE-labeled antibodies of the specificity of interest or with corresponding isotype-matched control antibodies at the dilution recommended by the supplier. When a non-labeled primary antibody was used, cells were washed after 30 minutes and incubated with a PE-labeled secondary antibody at 4°C. After 30 minutes, the cells were again washed with ice-cold PBS to remove unbound antibodies. Finally, cells were analyzed using a FACSCalibur (BD Biosciences, Heidelberg, GER) according to standard protocols. The following antibodies were used: PE-conjugated anti-hBaffR clone 11C1 (#558097, BD Biosciences), PE-conjugated anti-hBCMA clone 19F2 (#357504, Biolegend, San Diego, CA, USA), PE-conjugated anti-hCD40 clone HB14 (#130-094-135, Miltenyi Biotec, Bergisch Gladbach, DEU), anti-hCD95 clone DX2 (#MAB142, R&D Systems, Minneapolis, MN, USA), PE-conjugated anti-hTACI clone 165604 (#FAB1741P, R&D Systems), PE-conjugated anti-mIgG1 clone 11711 (#IC002P, R&D Systems), PE-conjugated anti-mIgG2a clone 20102 (#IC003P, R&D Systems), PE-conjugated anti-mIgG2b clone 133303 (#IC0041P, R&D Systems), PE-conjugated polyclonal anti-mIgG (#P9670, Sigma-Aldrich).
Equilibrium binding studies
Cells (5 x 105 cells in a microcentrifuge tube) transiently transfected with the TNFRSF receptor (TNFR) of interest or EV were incubated with increasing concentrations of the GpL-tagged antibody fusion proteins or ligands for 1 hour at 37°C. Cells were then washed three times with 1 ml ice-cold PBS, transferred to black 96-well plates and the cell-bound GpL activity was measured using the BioLux Gaussia Luciferase Assay Kit (#E3300, New England Biolabs) and a LUmo luminometer (Anthos Mikrosysteme GmbH, Friesoythe, Germany) according to the manufacturer’s recommendations. GpL activity of fusion proteins interacting with TNFR-transfected cells was considered as total binding, whereas the GpL activity associated with the EV-transfected cells was considered as nonspecific binding. Specific binding was calculated by subtraction of the nonspecific binding values from the corresponding total binding values. KD-values were fitted to a one-side specific binding plot by non-linear regression using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
Analysis of IL8 induction
CD40/CD95 responder cells (HT1080, HT1080-CD40, U2OS) were seeded in 96-well plates (2 x 104 cells/well) and were supplemented the next day with anchoring cells (Baff receptor or FcγR transfectants, Baff receptor-expressing cell lines) along with fresh medium containing the antibodies or antibody fusion proteins of interest. After an additional day, cell culture supernatants were analyzed with respect to their IL8 content using a human IL8 ELISA kit (BD Biosciences, Heidelberg, Germany) according to the instructions of the supplier. In experiments where TNC-Baff was used as competitor, this compound was added to the cells 30 minutes prior to the antibody constructs. When CD95-induced IL8 production was evaluated, cells were pre-incubated with the caspase inhibitor zVAD-fmk (20 µM; #4026865, Bachem, Bubendorf, Switzerland) to prevent cell death induction and with CHX (2.5 µg/ml) to enhance CD95 signaling.
Analysis of cellular viability
For evaluation of CD95-mediated induction of apoptosis, HT1080 cells were seeded in 96-well tissue culture plates (2 x 104 cells/well) and were treated the next day in triplicates with different concentrations of the various antibodies and antibody fusion proteins as well as the indicated anchoring cell population. If not indicated differently, stimulation was performed in the presence of CHX (2.5 µg/ml). After 20–24 hours, the cellular viability of cells in suspension was determined by means of the MTT assay and the viability of adherent cells was quantified by crystal violet staining as previously described by Lang et al.44 Viability values of untreated control cells (100%) and cells that were challenged with a toxic mixture (0%) were used for normalization.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 324392634 – TRR 221grant to H.W. and a M4-Award grant from the state Bavaria BIO-1601-0005 (A.B. and H.W.).
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) [324392634 – TRR 221]; State Bavaria [BIO-1601-0005].
Abbreviations
| AD AT ADC ADCC Baff BaffR BCMA DC DMEM EV FBS FcγRs GpL IL MM scBaff TCR TNF TNFRSF |
anchoring domain anchoring target antibody-drug conjugate antibody-dependent cell-mediated cytotoxicity B-cell activating factor Baff receptor B-cell maturation antigen dendritic cell Dulbecco’s Modified Eagle’s medium empty vector fetal bovine serum Fc gamma receptors Gaussia princeps luciferase interleukin multiple myeloma single-chain encoded B-cell activating factor T cell receptor tumor necrosis factor tumor necrosis factor receptor superfamily |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Moran AE, Kovacsovics-Bankowski M, Weinberg AD.. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–10. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchan SL, Dou L, Remer M, Booth SG, Dunn SN, Lai C, Semmrich M, Teige I, Martensson L, Penfold CA, et al. Antibodies to costimulatory receptor 4-1BB enhance anti-tumor immunity via T regulatory cell depletion and promotion of CD8 T cell effector function. Immunity. 2018;49:958–70.e7. doi: 10.1016/j.immuni.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 4.Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2019. doi: 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Villalba A, Llorens-Bobadilla E, Wollny D. CD95 in cancer: tool or target? Trends Mol Med. 2013;19:329–35. doi: 10.1016/j.molmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17:352–66. doi: 10.1038/nrc.2017.28. [DOI] [PubMed] [Google Scholar]
- 7.Zamagni E, Tacchetti P, Pantani L, Cavo M. Anti-CD38 and anti-SLAMF7: the future of myeloma immunotherapy. Expert Rev Hematol. 2018;11:423–35. doi: 10.1080/17474086.2018.1456331. [DOI] [PubMed] [Google Scholar]
- 8.Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Front Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensinger W, Maziarz RT, Jagannath S, Spencer A, Durrant S, Becker PS, Ewald B, Bilic S, Rediske J, Baeck J, et al. A phase 1 study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol. 2012;159:58–66. doi: 10.1111/j.1365-2141.2012.09251.x. [DOI] [PubMed] [Google Scholar]
- 10.Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, Harrop K, Drachman JG, Whiting N. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95:845–48. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locksley RM, Killeen N, Lenardo MJ, The TNF. TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 12.Medler J, Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opin Ther Targets. 2019;23:295–307. doi: 10.1080/14728222.2019.1586886. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H. Principles of antibody-mediated TNF receptor activation. Cell Death Differ. 2015;22:1727–41. doi: 10.1038/cdd.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piechutta M, Berghoff AS. New emerging targets in cancer immunotherapy: the role of cluster of differentiation 40 (CD40/TNFR5). ESMO Open. 2019;4:e000510. doi: 10.1136/esmoopen-2019-000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–09. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 16.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–30. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Szalai AJ, Zhou T, Zinn KR, Chaudhuri TR, Li X, Koopman WJ, Kimberly RP. Fc gamma Rs modulate cytotoxicity of anti-Fas antibodies: implications for agonistic antibody-based therapeutics. J Immunol. 2003;171:562–68. doi: 10.4049/jimmunol.171.2.562. [DOI] [PubMed] [Google Scholar]
- 18.Jodo S, Kung JT, Xiao S, Chan DV, Kobayashi S, Tateno M, Lafyatis R, Ju ST. Anti-CD95-induced lethality requires radioresistant Fcgamma RII+ cells. A novel mechanism for fulminant hepatic failure. J Biol Chem. 2003;278:7553–57. doi: 10.1074/jbc.M211229200. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–34. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Ravetch JV. Antitumor activities of agonistic anti-TNFR antibodies require differential FcgammaRIIB coengagement in vivo. Proc Natl Acad Sci USA. 2013;110:19501–06. doi: 10.1073/pnas.1319502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcgammaR engagement. Cancer Cell. 2016;29:820–31. doi: 10.1016/j.ccell.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark EA, Yip TC, Ledbetter JA, Yukawa H, Kikutani H, Kishimoto T, Ng MH. CDw40 and BLCa-specific monoclonal antibodies detect two distinct molecules which transmit progression signals to human B lymphocytes. Eur J Immunol. 1988;18:451–57. doi: 10.1002/eji.1830180320. [DOI] [PubMed] [Google Scholar]
- 23.Chodorge M, Zuger S, Stirnimann C, Briand C, Jermutus L, Grutter MG, Minter RR. A series of Fas receptor agonist antibodies that demonstrate an inverse correlation between affinity and potency. Cell Death Differ. 2012;19:1187–95. doi: 10.1038/cdd.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 25.Tai YT, Anderson KC. B cell maturation antigen (BCMA)-based immunotherapy for multiple myeloma. Expert Opin Biol Ther. 2019;19:1143–56. doi: 10.1080/14712598.2019.1641196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francisco JA, Donaldson KL, Chace D, Siegall CB, Wahl AF. Agonistic properties and in vivo antitumor activity of the anti-CD40 antibody SGN-14. Cancer Res. 2000;60:3225–31. [PubMed] [Google Scholar]
- 27.Tai YT, Li X, Tong X, Santos D, Otsuki T, Catley L, Tournilhac O, Podar K, Hideshima T, Schlossman R, et al. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res. 2005;65:5898–906. doi: 10.1158/0008-5472.Can-04-4125. [DOI] [PubMed] [Google Scholar]
- 28.Horton HM, Bernett MJ, Peipp M, Pong E, Karki S, Chu SY, Richards JO, Chen H, Repp R, Desjarlais JR, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116:3004–12. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- 29.Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG, Mixan BJ, Lee WP, Lin Z, Valdez P, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–38. doi: 10.1158/0008-5472.Can-05-0095. [DOI] [PubMed] [Google Scholar]
- 30.Askenasy N, Yolcu ES, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105:1396–404. doi: 10.1182/blood-2004-06-2364. [DOI] [PubMed] [Google Scholar]
- 31.Igney FH, Behrens CK, Krammer PH. Tumor counterattack–concept and reality. Eur J Immunol. 2000;30:725–31. doi:. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–60. doi: 10.1158/1078-0432.Ccr-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreaux J, Hose D, Jourdan M, Reme T, Hundemer M, Moos M, Robert N, Moine P, De Vos J, Goldschmidt H, et al. TACI expression is associated with a mature bone marrow plasma cell signature and C-MAF overexpression in human myeloma cell lines. Haematologica. 2007;92:803–11. doi: 10.3324/haematol.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA, Greipp PR, Jelinek DF. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–94. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 35.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, Richardson PG, Anderson LD Jr., Sutherland HJ, Yong K, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641–53. doi: 10.1016/s1470-2045(18)30576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, Richardson PG, Hoos A, Gupta I, Bragulat V, et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9:37. doi: 10.1038/s41408-019-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267–80. doi: 10.1200/jco.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K, Nelson A, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129:2210–21. doi: 10.1172/jci126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, van Leeuwen DG, Purdon T, Pegram HJ, Brentjens RJ. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–78. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn NF, Purdon TJ, van Leeuwen DG, Lopez AV, Curran KJ, Daniyan AF, Brentjens RJ. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell. 2019;35:473–88.e6. doi: 10.1016/j.ccell.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyzgol A, Muller N, Fick A, Munkel S, Grigoleit GU, Pfizenmaier K, Wajant H. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J Immunol. 2009;183:1851–61. doi: 10.4049/jimmunol.0802597. [DOI] [PubMed] [Google Scholar]
- 44.Lang I, Fullsack S, Wyzgol A, Fick A, Trebing J, Arana JA, Schafer V, Weisenberger D, Wajant H. Binding studies of TNF receptor superfamily (TNFRSF) receptors on intact cells. J Biol Chem. 2016;291:5022–37. doi: 10.1074/jbc.M115.683946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kums J, Nelke J, Ruth B, Schafer V, Siegmund D, Wajant H. Quantitative analysis of cell surface antigen-antibody interaction using Gaussia princeps luciferase antibody fusion proteins. MAbs. 2017;9:506–20. doi: 10.1080/19420862.2016.1274844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


