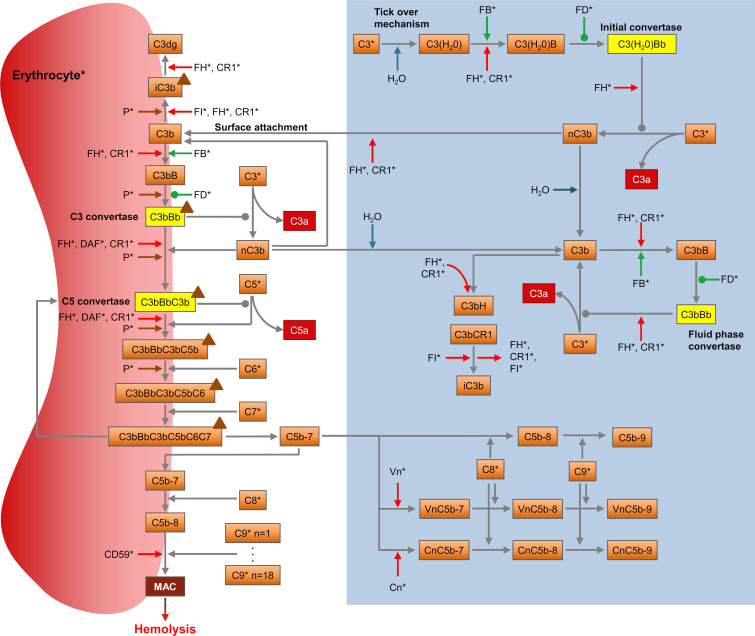
Fig 1. The alternative pathway of the complement system.
The diagram shows the pathway as described in the model with its components (orange boxes), reactions (grey arrows), proactivators (green arrows), regulators (red arrows), convertases (yellow boxes), effectors (red boxes), and the positive regulator properdin (brown triangles). Association/dissociation reactions are displayed with a pointed arrowhead, enzymatic reactions with an oval. Degradation was implemented for all proteins and complexes. Proteins marked with a (*) are synthesized. The blue box indicates the reactions of the fluid phase in absence of erythrocytes. Activation of the AP begins with spontaneous hydrolysis (“tick-over”) of C3 producing C3(H2O), which in turn can form the initial convertase C3(H2O)Bb in presence of FB and factor D (FD). The initial convertase can generate more C3b through C3 cleavage. C3b may bind FB, leading to the formation of the fluid-phase convertase C3bBb, or attach to a nearby surface, such as a cell membrane. Surface-attached C3b can combine with FB to form the surface C3 convertase C3bBb, reacting with C3b to generate the C5 convertase, C3bBbC3b. Cleavage of C5 by the C5 convertase activates the terminal pathway. The anaphylatoxin C5a is released while C5b remains attached to the C5 convertase, followed by C6 and C7 binding. The complex C5b-7 is released into the fluid phase, from where it can reinsert into the cell membrane if not sequestered by the regulators vitronectin (Vn) or clusterin (Cn). Upon binding of membrane C5b-7 to C8 and up to 18 C9 molecules [42], the membrane attack complex (MAC) is formed. With sufficient terminal pathway activity, the cell is lysed as a result of the accumulation of MAC complexes, permitting free diffusion of molecules across its membrane. All negative regulators (FH, decay-accelerating factor (DAF), CD59, complement receptor type 1 (CR1), factor I (FI), Vn and Cn) are included in the model, as well as the only known positive regulator, properdin (P), which can stabilize the C3 and C5 convertase.