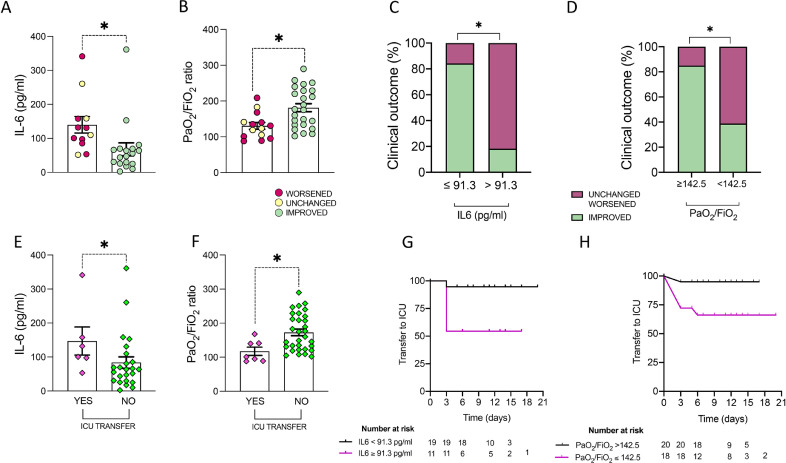
Fig. 2.
A-H. Clinical and biological baseline predictors of clinical outcome in SARS-Cov-2pos patients treated with Sarilumab. A) Baseline IL-6 plasma levels in SARS-Cov-2pos patients treated with Sarilumab based on clinical improvement at 3 days after Sarilumab treatment, *p = 0.001. B) Baseline PaO2/FiO2 in SARS-Cov-2pos patients treated with Sarilumab based on clinical improvement at 3 days after Sarilumab treatment, *p = 0.004. C) Rate of clinical improvement at 3 days in SARS-Cov-2pos patients treated with Sarilumab based on baseline IL-6 plasma levels, *p < 0.0001. D) Rate of clinical improvement at 3 days in SARS-Cov-2pos patients treated with Sarilumab based on baseline PaO2/FiO2, p = 0.003. E) Baseline IL-6 plasma levels in SARS-Cov-2pos patients based on transfer towards ICU within 14 days after Sarilumab treatment, *p = 0.05. F) Baseline PaO2/FiO2 in SARS-Cov-2pos patients based on transfer towards ICU within 14 days after Sarilumab treatment, *p = 0.008. G) Kaplan-Meier analysis of transfer to ICU rate in SARS-Cov-2pos patients treated with Sarilumab based on pre-treatment IL-6 plasma levels, p = 0.01. H) Kaplan-Meier analysis of transfer to ICU rate in SARS-Cov-2pos patients treated with Sarilumab based on pre-treatment PaO2/FiO2, p = 0.03.