Fig. 7. Biological response to PMMA-encapsulated quantum dots.
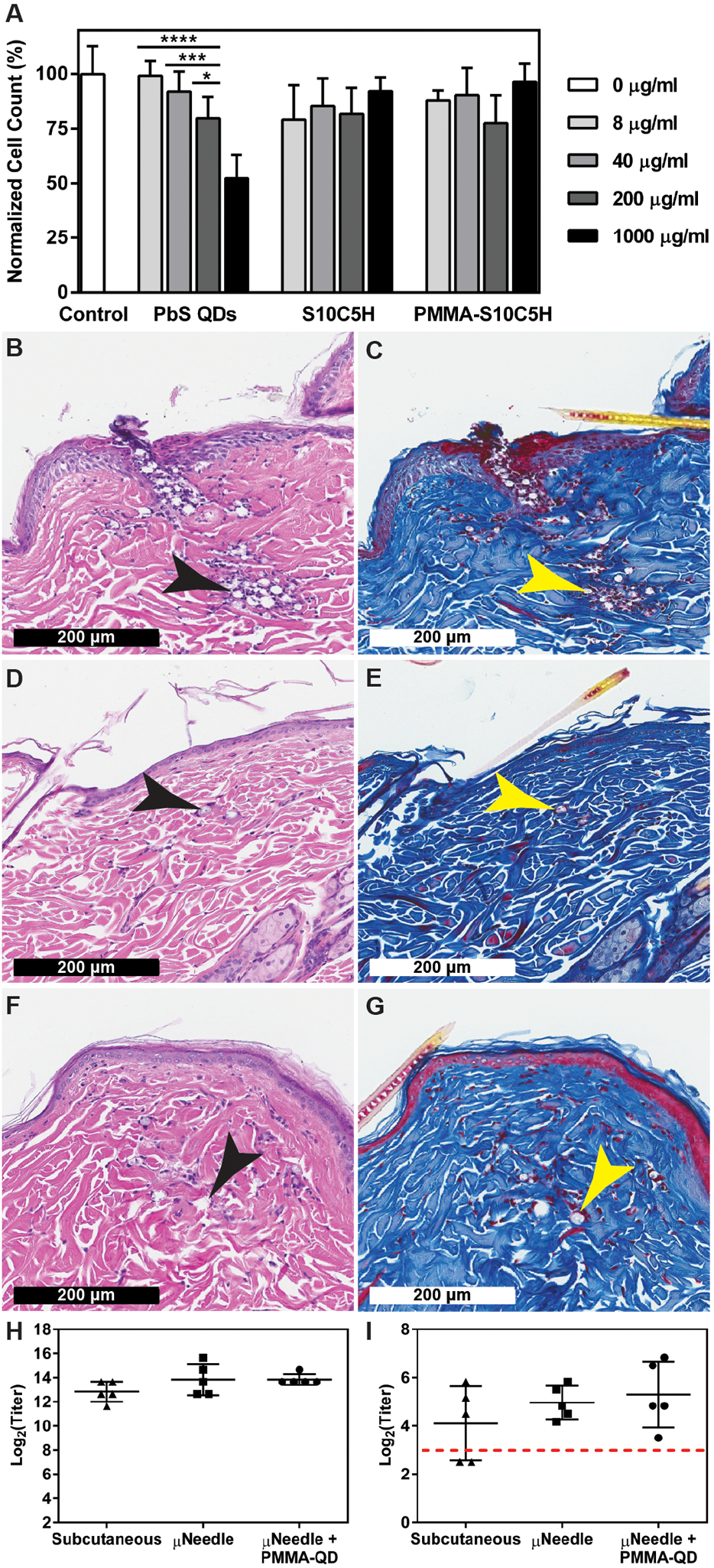
(A) In vitro cytotoxicity of commercially-available PbS QDs compared to unencapsulated and PMMA-encapsulated S10C5H QDs over 24 hours in a mouse macrophage cell line (Raw 264.7). n=3, *P < 0.05, ***P < 0.001, ****P < 0.0001 (two-way ANOVA with Tukey’s multiple comparisons). Representative histological samples collected from rats receiving microneedle-delivered PMMA particles containing S10C5H QDs (B, C) one day, (D, E) two weeks, and (F, G) four weeks after administration stained with hematoxylin and eosin or Masson’s trichrome, respectively. Arrows indicate the location of microparticles. (H) Total anti-poliovirus type 2 IgG antibody titers and (H) neutralizing poliovirus type 2 antibody titers showing no differences after three doses of type 2 inactivated poliovirus vaccine delivered via subcutaneous injections or microneedles with or without PMMA-encapsulated QDs, n=5. Dashed line indicates the threshold above which humans are considered immune.
