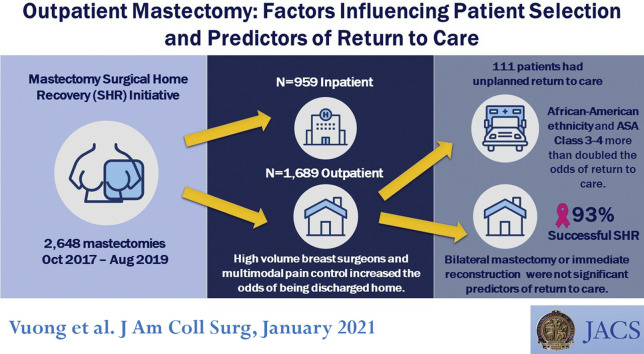
Abstract
Background
After implementation of the Surgical Home Recovery (SHR) initiative for mastectomy within a large, integrated health delivery system, most patients are discharged on the day of the procedure. We sought to identify predictors of SHR and unplanned return to care (RTC).
Study Design
Mastectomy cases with and without reconstruction from October 2017 to August 2019 were analyzed. Patient characteristics, operative variables, and multimodal pain management were compared between admitted patients and SHR patients using logistic regression. We identified predictors of RTC in SHR patients, defined as 7-day readmission, reoperation, or emergency department visit.
Results
Of 2,648 mastectomies, 1,689 (64%) were outpatient procedures and the mean age of patients was 58.5 years. Predictors of SHR included perioperative IV acetaminophen (odds ratio [OR] 1.59; 95% CI, 1.28 to 1.97), perioperative opiates (OR 1.47; 95% CI, 1.06 to 2.02), and operation performed by a high-volume breast surgeon (OR 2.12; 95% CI, 1.42 to 3.18). Bilateral mastectomies (OR 0.70; 95% CI, 0.54 to 0.91), immediate reconstruction (OR 0.52; 95% CI, 0.39 to 0.70), and American Society of Anesthesiologists class 3 to 4 (OR 0.69; 95% CI, 0.54 to 0.87) decreased the odds of SHR. Of SHR patients, 111 of 1,689 patients (7%) experienced RTC. Patients with American Society of Anesthesiologists class 3 to 4 (OR 2.01; 95% CI, 1.29 to 3.14) and African American race (OR 2.30; 95% CI, 1.38 to 4.91) were more likely to RTC; receiving IV acetaminophen (OR 0.56; 95% CI, 0.35 to 0.88) and filling an opiate prescription (OR 0.51; 95% CI, 0.34 to 0.77) decreased the odds of RTC.
Conclusions
Surgeon volume and multimodal pain medication increased the odds of SHR. Within the SHR group, American Society of Anesthesiologists Class 3 to 4 and African American patients increased the likelihood of RTC. This study helps optimize patient selection and perioperative practice for successful SHR.
Abbreviations and Acronyms: ASA, American Society of Anesthesiologists physical status; ERAS, Enhanced Recovery after Surgery; LOS, length of stay; OM, outpatient mastectomy; RTC, return to care; SHR, Surgical Home Recovery; TRAS, Traditional Recovery after Surgery
Visual Abstract
Critics of same-day discharge for mastectomy have denounced it as “drive-through” care.1 Nonetheless, over time and with more robust support services, the physical and psychological benefits of early discharge after breast operation have been recognized.2 Rising mastectomy rates across the US,3 coupled with the recent COVID-19 pandemic, have driven healthcare systems to deliver efficient, effective, and safe care to breast cancer patients. Currently, one-third of bilateral mastectomies and one-half of unilateral mastectomies are performed in the outpatient setting.4 Same-day discharge for mastectomy patients mitigates potential harms and costs associated with an inpatient admission, conserves valuable hospital resources, and affords patients postoperative recovery in the comfort of their own homes without any increase in complication rates. Despite multiple retrospective series concluding that outpatient mastectomy is safe,5, 6, 7, 8 adoption of this practice varies nationwide.4 , 9 , 10
We previously published our organization’s experience with the implementation of the Surgical Home Recovery (SHR) initiative, which enabled the rapid transition from predominantly inpatient to predominantly outpatient mastectomies.11 The SHR initiative was a coordinated and systematic approach, using a variety of interventions starting with preoperative patient education including drain management, staff and surgeon support for same-day discharge, applying Enhanced Recovery After Surgery (ERAS) principles of multimodal pain management, and close postoperative follow-up. In addition, we found that complication rates were low for all mastectomy patients and, more importantly, the complication rates were the same whether a patient went home the same day as the procedure or remained in the hospital overnight.
Because many interventions occurred simultaneously to achieve SHR, it is unclear which interventions were most meaningful to drive these changes. In this article, we evaluate independent factors, including patient characteristics, operative details, and multimodal pain management, to determine which variables are more strongly associated with SHR. Focusing on SHR patients, we analyzed predictors of unplanned return to care (RTC). Identifying variables that facilitate mastectomy SHR and recognizing factors that place these patients at higher risk for RTC will help surgeons and healthcare systems provide patients with safe and appropriate recovery after mastectomy.
Methods
Kaiser Permanente Northern California provides comprehensive care for more than 4.3 million members across 21 medical centers. In October 2017, Kaiser Permanente Northern California implemented SHR for patients undergoing mastectomy. This was a retrospective review of a prospectively collected database from the electronic health record. This study evaluated all members undergoing unilateral or bilateral mastectomy, including immediate tissue-expander or implant-based reconstruction, between October 2017 and August 2019. The main study end point was SHR, which was defined as the patient being discharged from the hospital on the same calendar day as mastectomy. Among patients who underwent SHR, we assessed RTC. This was defined as any unplanned reoperation, readmission, or emergency department visit for any reason within 7 days of mastectomy. The reason for RTC was determined by manual chart review performed by the surgical authors (BV, GK, SC).
Patients with microvascular free flap reconstruction or other nonbreast procedures on the same day as mastectomy were excluded. Patients who were male or undergoing operations for gynecomastia or gender reassignment were also excluded. The reason for operation was determined from text analysis of procedure names, diagnoses associated with the procedure, and chart review. Covariables included patient-level characteristics (age, race/ethnicity, BMI, neoadjuvant chemotherapy, American Society of Anesthesiologists physical status [ASA] class),12 operative factors (procedure laterality, reconstruction, nipple-sparing technique, axillary node dissection or sentinel node biopsy, length of operation, estimated blood loss, whether the procedure was performed by a high-volume breast surgeon), and multimodal pain management (delta pain score, NSAIDs, liposomal bupivacaine, gabapentin use, IV lidocaine, IV acetaminophen, ketamine injection, locoregional nerve block, opiates).
BMI was the latest measurement on or up to 1 year before date of operation. Neoadjuvant chemotherapy was defined as any chemotherapy for breast cancer in the year before mastectomy. Reconstruction, nipple-sparing technique, axillary operation, and estimated blood loss were determined through text analysis of procedure names and operative reports. Surgeons who performed 50 or more breast cases per year were categorized as high-volume.
Delta pain score was the mean difference between patient-reported pain on a 0 to 10 visual scale and patient-reported acceptable level of pain throughout all measurements taken on the day of mastectomy.13 A lower number indicates that the patient-reported pain was lower than the acceptable level of pain, translating into more favorable pain control. Perioperative medications were defined as being received between 4 hours before operation through the end of the day of mastectomy. In addition, the second model (RTC) included discharge opioids filled between discharge and RTC or end of follow-up (7 days after mastectomy).
Chi-square analysis and Fisher exact tests for categorical variables and t-tests for continuous variables were used to compare the clinical and perioperative patient characteristics of the inpatient vs SHR groups and RTC vs no RTC groups. A p value < 0.05 was considered significant. Two separate multivariable logistic regression analyses were performed to identify predictors of SHR and RTC. SAS Software, version 9.4 (SAS Institute) was used to analyze the data. To account for clustering of physician practices by medical center, we performed generalized linear mixed models with a binomial distribution and logit link function. There was minimal clustering by medical center in the model predicting RTC, so clustering was removed from the final model. We adjusted both models for the month of the study in which the mastectomy was performed to account for changing discharge practices over time.14 To assess the relative contribution of patient, operative, and multimodal pain management variables, we added each group of variables to models in a stepwise fashion. However, only the final complete model is shown, as the models did not differ substantially when adding groups of variables. The Research Determination Committee for the Kaiser Permanente North California region has determined the project does not meet regulatory definition of research involving human subjects per 45 CFR 46.102(f).15
Results
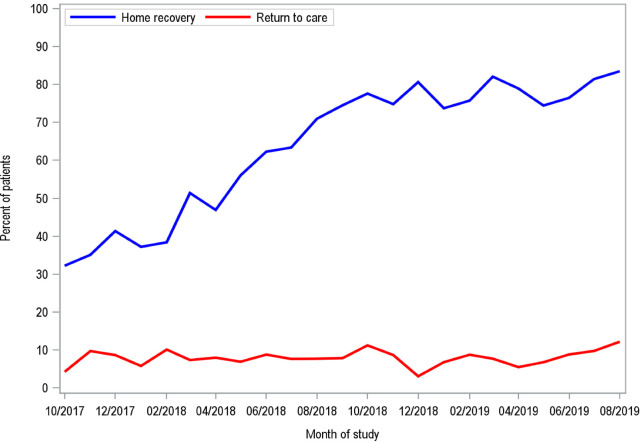
Between October 2017 and August 2019, there were 3,529 mastectomies performed. We excluded 284 mastectomies ineligible for home recovery due to autologous flap reconstruction or simultaneous nonbreast operation, and 597 mastectomies performed for gynecomastia or gender reassignment, leaving 2,648 mastectomies available for analysis. SHR was carried out for 1,689 patients (64%) (Table 1 ), of which only 111 patients had RTC (Table 2 ). The mean length of stay (LOS) for inpatient mastectomies was 28.0 hours, compared with 3.2 hours for outpatient mastectomies. During the course of the study period, more patients experienced SHR with no significant increase in the rate of RTC (Fig. 1 ).
Table 1.
Characteristics of 2,648 Kaiser Permanente Northern California Mastectomy Procedures, October 2017 to August 2019
| Characteristic | Inpatient (n = 959) | Home recovery (n = 1,689) | p Value |
|---|---|---|---|
| Patient characteristic | |||
| Age, n (%) | 0.02 | ||
| 18–39 y | 103 (11) | 128 (8) | |
| 40–64 y | 541 (56) | 988 (59) | |
| ≥ 65 y | 315 (33) | 573 (34) | |
| Age, y, mean (SD) | 58.2 (14.3) | 58.7 (13.5) | 0.42 |
| Race/ethnicity, n (%) | < 0.01 | ||
| African American | 51 (5) | 102 (6) | |
| Asian American | 161 (17) | 380 (23) | |
| Hispanic | 143 (15) | 231 (14) | |
| White | 554 (58) | 872 (52) | |
| Other/missing | 50 (5) | 104 (6) | |
| Charlson Comorbidity Index, n (%) | 0.14 | ||
| 0–3 | 582 (61) | 1,080 (64) | |
| 4–8 | 340 (35) | 561 (33) | |
| ≥ 9 | 37 (4) | 48 (3) | |
| BMI, n (%) | 0.48 | ||
| < 18.5 kg/m2 | 20 (2) | 45 (3) | |
| 18.5–24.9 kg/m2 | 364 (38) | 657 (39) | |
| 25.0–29.9 kg/m2 | 286 (30) | 520 (31) | |
| ≥ 30 kg/m2 | 289 (30) | 467 (28) | |
| Neoadjuvant chemotherapy, n (%) | 0.84 | ||
| Yes | 129 (13) | 232 (14) | |
| No | 830 (87) | 1457 (86) | |
| Operative factor | |||
| LOS post operation, h, mean (SD) | 28.0 (19.8) | 3.2 (1.4) | < 0.01 |
| ASA class, n (%) | 0.10 | ||
| 1 | 46 (5) | 70 (4) | |
| 2 | 575 (60) | 1084 (64) | |
| 3 | 325 (34) | 522 (31) | |
| 4 | 13 (1) | 13 (< 1) | |
| Operation, n (%) | < 0.01 | ||
| Unilateral with reconstruction | 209 (22) | 327 (19) | |
| Unilateral without reconstruction | 377 (39) | 969 (57) | |
| Bilateral with reconstruction | 252 (26) | 190 (11) | |
| Bilateral without reconstruction | 121 (13) | 203 (12) | |
| Nipple-sparing technique, n (%) | < 0.01 | ||
| Yes | 190 (20) | 255 (15) | |
| No | 769 (80) | 1434 (85) | |
| Nodal operation, n (%) | 0.35 | ||
| Axillary node dissection | 416 (43) | 684 (41) | |
| Sentinel node biopsy | 364 (38) | 674 (40) | |
| None | 179 (19) | 331 (20) | |
| Operating time, n (%) | < 0.01 | ||
| < 2 h | 294 (31) | 836 (50) | |
| 2–4 h | 474 (49) | 737 (44) | |
| > 4 h | 191 (20) | 116 (7) | |
| Estimated blood loss, n (%) | < 0.01 | ||
| < 100 mL | 853 (89) | 1,600 (95) | |
| ≥ 100 mL | 98 (10) | 56 (3) | |
| Missing | 8 (< 1) | 33 (2) | |
| High-volume breast surgeon, n (%) | < 0.01 | ||
| Yes | 844 (88) | 1,615 (96) | |
| No | 115 (12) | 74 (4) | |
| Multimodal pain management | |||
| Delta pain score, mean (SD) | –0.9 (1.9) | –1.8 (2.1) | < 0.01 |
| NSAID, n (%) | 153 (16) | 213 (13) | 0.02 |
| Liposomal bupivacaine, n (%) | 80 (8) | 177 (10) | 0.07 |
| Gabapentin, n (%) | 358 (37) | 527 (31) | < 0.01 |
| IV lidocaine, n (%) | 32 (3) | 38 (2) | 0.09 |
| IV acetaminophen, n (%) | 569 (59) | 978 (58) | 0.47 |
| Ketamine, n (%) | 134 (14) | 279 (17) | 0.08 |
| Nerve block, n (%) | 227 (24) | 485 (29) | < 0.01 |
| Any opioids, n (%) | 816 (85) | 1501 (89) | < 0.01 |
ASA, American Society of Anesthesiologists physical status; LOS, length of stay.
Table 2.
Characteristics of 1,689 Kaiser Permanente Northern California Mastectomy Patients Who Underwent Home Recovery, October 2017 to August 2019
| Characteristic | Successful home recovery (n = 1,578) | Return to care (n = 111) | p Value |
|---|---|---|---|
| Patient characteristic | |||
| Age, n (%) | 0.67 | ||
| 18–39 y | 122 (8) | 6 (5) | |
| 40–64 y | 921 (58) | 67 (60) | |
| ≥ 65 y | 535 (34) | 38 (34) | |
| Age, y, mean (SD) | 58.6 (13.5) | 59.8 (13.3) | 0.38 |
| Race/ethnicity, n (%) | < 0.01 | ||
| African American | 85 (5) | 17 (15) | |
| Asian American | 361 (23) | 19 (17) | |
| Hispanic | 215 (14) | 16 (14) | |
| White | 818 (52) | 54 (49) | |
| Other/missing | 99 (6) | — | |
| Charlson Comorbidity Index, n (%) | < 0.01 | ||
| 0–3 | 1,022 (65) | 58 (52) | |
| 4–8 | 508 (32) | 53 (48) | |
| ≥ 9 | 48 (3) | — | |
| BMI, n (%) | 0.10 | ||
| < 18.5 kg/m2 | 43 (3) | — | |
| 18.5–24.9 kg/m2 | 625 (40) | 32 (29) | |
| 25.0–29.9 kg/m2 | 482 (31) | 38 (34) | |
| ≥ 30 kg/m2 | 428 (27) | 39 (35) | |
| Neoadjuvant chemotherapy, n (%) | 0.43 | ||
| Yes | 214 (14) | 18 (16) | |
| No | 1,364 (86) | 93 (84) | |
| Operative factor | |||
| LOS postoperative, h, mean (SD) | 3.2 (1.4) | 3.3 (1.4) | 0.79 |
| ASA class, n (%) | < 0.01 | ||
| 1 | 63 (4) | 7 (6) | |
| 2 | 1,034 (66) | 50 (45) | |
| 3 | 469 (30) | 53 (48) | |
| 4 | 12 (< 1) | — | |
| Operation, n (%) | 0.94 | ||
| Unilateral with reconstruction | 308 (20) | 19 (17) | |
| Unilateral without reconstruction | 904 (57) | 65 (59) | |
| Bilateral with reconstruction | 177 (11) | 13 (12) | |
| Bilateral without reconstruction | 189 (12) | 14 (13) | |
| Nipple-sparing technique, n (%) | 0.19 | ||
| Yes | 243 (15) | 12 (11) | |
| No | 1,335 (85) | 99 (89) | |
| Nodal surgery, n (%) | 0.38 | ||
| Axillary node dissection | 637 (40) | 47 (42) | |
| Sentinel node biopsy | 636 (40) | 38 (34) | |
| None | 305 (19) | 26 (23) | |
| Operating time, n (%) | 0.13 | ||
| < 2 h | 772 (49) | 64 (58) | |
| 2–4 h | 694 (44) | 43 (39) | |
| > 4 h | 112 (7) | — | |
| Estimated blood loss, n (%) | 0.83 | ||
| < 100 mL | 1,494 (95) | 106 (96) | |
| ≥ 100 mL | 52 (3) | — | |
| Missing | 32 (2) | — | |
| High-volume breast surgeon, n (%) | 0.95 | ||
| Yes | 1,509 (96) | 106 (96) | |
| No | 69 (4) | — | |
| Multimodal pain management | |||
| Delta pain score, mean (SD) | –1.8 (2.1) | –1.6 (2.0) | 0.23 |
| NSAID, n (%) | 200 (13) | 13 (12) | 0.77 |
| Liposomal bupivacaine, n (%) | 163 (10) | 14 (13) | 0.46 |
| Gabapentin, n (%) | 491 (31) | 36 (32) | 0.77 |
| IV lidocaine, n (%) | 34 (2) | — | 0.31 |
| IV acetaminophen, n (%) | 923 (58) | 55 (50) | 0.07 |
| Ketamine, n (%) | 259 (16) | 20 (18) | 0.66 |
| Nerve block, n (%) | 450 (29) | 35 (32) | 0.50 |
| Any opioid, n (%) | 1,408 (89) | 93 (84) | 0.08 |
| Filled opioid post discharge, n (%) | < 0.01 | ||
| Yes | 813 (52) | 41 (37) | |
| No | 765 (48) | 70 (63) |
ASA, American Society of Anesthesiologists physical status; LOS, length of stay.
Figure 1.
Rate of home recovery and return to care from October 2017 to August 2019.
Surgical home recovery
Home recovery and inpatients had similar baseline characteristics except that patients with home recovery were more likely to be Asian American (23% SHR vs 17% inpatient) and less likely to be White (52% SHR vs 58% inpatient; p < 0.01). There was no difference in patients undergoing neoadjuvant chemotherapy, with 14% of the SHR population receiving neoadjuvant chemotherapy. More than half (57%) of the SHR population underwent unilateral mastectomy without reconstruction. There were 517 patients who were discharged after reconstruction; of these, 327 were unilateral and 190 bilateral. Fewer patients undergoing nipple-sparing mastectomy were discharged the same day (15% SHR vs 20% inpatient; p < 0.01). Nodal operation was performed in 80% of the home recovery patients. A vast majority (96%) of the SHR patients had their procedure performed by a high-volume breast surgeon. Opiates (89%), IV acetaminophen (58%), and gabapentin (31%) were the most common perioperative modalities of pain management among the SHR group. The delta pain score was lower in the SHR group (–1.8 SHR vs –0.9 inpatient; p < 0.01).
In the adjusted model for SHR (vs inpatient mastectomy), increased age (odds ratio [OR] 0.98; 95% CI, 0.97 to 0.99), ASA class 3 to 4 (OR 0.69; 95% CI, 0.54 to 0.87), bilateral operation (OR 0.70; 95% CI, 0.54 to 0.91), immediate reconstruction (OR 0.52; 95% CI, 0.39 to 0.70), estimated blood loss more than 100 mL (OR 0.36; 95% CI, 0.23 to 0.57), perioperative NSAIDs (OR 0.50; 95% CI, 0.37 to 0.67), and perioperative gabapentin (OR 0.49; 95% CI, 0.38 to 0.63) were all associated with lower odds of same-day discharge (Table 3 , Model 1). Having a high-volume breast surgeon (OR 2.12; 95% CI, 1.42 to 3.18), perioperative IV acetaminophen (OR 1.59; 95% CI, 1.28 to 1.97), and perioperative opioids (OR 1.47; 95% CI, 1.06 to 2.02) increased the odds of home recovery.
Table 3.
Multivariate Logistic Regression Models for (Model 1) Surgical Home Recovery and (Model 2) Return to Care
| Characteristic | Model 1: Home recovery∗ (n = 2,642) |
Model 2: Return to care† (n = 1,686) |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Patient characteristic | ||||||
| Age, y | 0.98 | 0.97–0.99 | < 0.01 | 1.00 | 0.98-1.02 | 0.75 |
| Race/ethnicity | ||||||
| African American | 0.79 | 0.50–1.24 | 0.30 | 2.60 | 1.38–4.91 | < 0.01 |
| Asian American | 1.00 | 0.75–1.33 | 1.00 | 0.92 | 0.52–1.63 | 0.77 |
| Hispanic | 0.75 | 0.55–1.02 | 0.06 | 1.19 | 0.65–2.19 | 0.57 |
| White | 1.00 | Ref | 1.00 | Ref | ||
| Other/missing | 0.83 | 0.53–1.31 | 0.43 | 0.69 | 0.26–1.80 | 0.45 |
| BMI | ||||||
| < 18.5 kg/m2 | 0.81 | 0.43–1.52 | 0.51 | 0.68 | 0.15–3.12 | 0.62 |
| 18.5–24.9 kg/m2 | 1.00 | Ref | 1.00 | Ref | ||
| 25.0–29.9 kg/m2 | 1.01 | 0.79–1.29 | 0.93 | 1.23 | 0.76–2.09 | 0.37 |
| ≥ 30 kg/m2 | 0.88 | 0.68–1.15 | 0.36 | 1.25 | 0.73–2.15 | 0.41 |
| Neoadjuvant chemotherapy | 0.80 | 0.60–1.09 | 0.15 | 1.21 | 0.68–2.16 | 0.51 |
| Operative factor | ||||||
| ASA class | ||||||
| 1–2 | 1.00 | Ref | 1.00 | Ref | ||
| 3–4 | 0.69 | 0.54–0.87 | < 0.01 | 2.01 | 1.29–3.14 | < 0.01 |
| Laterality | ||||||
| Unilateral | 1.00 | Ref | 1.00 | Ref | ||
| Bilateral | 0.70 | 0.54–0.91 | < 0.01 | 1.42 | 0.83–2.43 | 0.20 |
| Reconstruction | 0.52 | 0.39–0.70 | < 0.01 | 1.45 | 0.80–2.64 | 0.22 |
| Nipple–sparing | 1.23 | 0.89–1.71 | 0.21 | 0.84 | 0.39–1.79 | 0.65 |
| Nodal operation | ||||||
| Axillary node dissection | 0.84 | 0.64–1.12 | 0.23 | 0.92 | 0.53–1.58 | 0.76 |
| Sentinel node biopsy | 1.10 | 0.83–1.45 | 0.51 | 0.81 | 0.47–1.41 | 0.46 |
| None | 1.00 | Ref | 1.00 | Ref | ||
| Operating time | ||||||
| < 2 h | 1.00 | Ref | 1.00 | Ref | ||
| 2–4 h | 0.55 | 0.41–0.74 | < 0.01 | 0.59 | 0.34–1.03 | 0.06 |
| > 4 h | 0.26 | 0.16–0.43 | < 0.01 | 0.26 | 0.07–0.91 | 0.04 |
| Estimated blood loss | ||||||
| < 100 mL | 1.00 | Ref | 1.00 | Ref | ||
| ≥ 100 mL | 0.36 | 0.23–0.57 | < 0.01 | 1.25 | 0.40–3.84 | 0.70 |
| Missing | 1.86 | 0.72–4.75 | 0.20 | 0.56 | 0.07–4.30 | 0.58 |
| High–volume breast surgeon | 2.12 | 1.42–3.18 | < 0.01 | 1.08 | 0.40–2.89 | 0.88 |
| Multimodal pain management | ||||||
| Delta pain score | 0.80 | 0.60–1.09 | 0.15 | 1.07 | 0.97–1.18 | 0.15 |
| NSAID | 0.50 | 0.37–0.67 | < 0.01 | 0.92 | 0.48–1.73 | 0.78 |
| Liposomal bupivacaine | 1.18 | 0.82–1.71 | 0.38 | 1.46 | 0.75–2.82 | 0.26 |
| Gabapentin | 0.49 | 0.38–0.63 | < 0.01 | 1.05 | 0.66–1.68 | 0.84 |
| IV lidocaine | 0.91 | 0.49–1.70 | 0.77 | 1.90 | 0.62–5.87 | 0.26 |
| IV acetaminophen | 1.59 | 1.28–1.97 | < 0.01 | 0.56 | 0.35–0.88 | 0.01 |
| Ketamine | 0.79 | 0.58–1.09 | 0.15 | 1.47 | 0.84–2.57 | 0.18 |
| Nerve block | 1.18 | 0.91–1.53 | 0.21 | 1.06 | 0.66–1.69 | 0.82 |
| Any opioid | 1.47 | 1.06–2.02 | 0.02 | 0.55 | 0.30–1.00 | 0.05 |
| Filled opioid post discharge | — | — | — | 0.51 | 0.34-0.77 | < 0.01 |
ASA, American Society of Anesthesiologists; OR, odds ratio; Ref, reference.
Model 1: Home recovery excludes 6 patients with missing delta pain score.
Model 2: Return to care excludes 3 patients with missing delta pain score.
Unplanned return to care
RTC was defined as an unplanned emergency department visit, readmission, or reoperation within 1 week of the date of operation. There were 111 patients who had to RTC, translating to 93% success for SHR. Among patients with home recovery, African American patients (15% RTC vs 5% successful home recovery; p < 0.01) and patients with more comorbidities were more likely to RTC.
Neoadjuvant chemotherapy, immediate reconstruction, or bilateral operation did not increase the odds of RTC (Table 3, Model 2). Operative time longer than 4 hours (OR 0.26; 95% CI, 0.07 to 0.91), perioperative acetaminophen (OR 0.56; 95% CI, 0.35 to 0.88), and a filled opioid discharge prescription (OR 0.51; 95% CI, 0.34 to 0.77) were associated with lower odds of RTC.
Of the 111 RTC patients, 65 patients (59%) presented to the emergency department and were subsequently discharged, 19 (17%) were readmitted, and 27 (24%) underwent reoperation (Table 4 ). The most common reasons for RTC included wound checks, concern for bleeding, and drain concerns. The overall reoperation rate for hematoma evacuation was 1.6% (27 of 1,689) for SHR patients.
Table 4.
Reasons for Return to Care Among Home Recovery Patients
| Variable | n | % of RTC (n = 111) | % of SHR (n = 1,689) |
|---|---|---|---|
| Highest level of return to care | |||
| Reoperation | 27 | 24.3 | 1.6 |
| Inpatient readmission | 19 | 17.1 | 1.1 |
| Emergency department | 65 | 58.6 | 3.8 |
| Reason for return to care | |||
| Bleeding/hematoma | 31 | 27.9 | 1.8 |
| Drain concern | 26 | 23.4 | 1.5 |
| Wound check | 14 | 12.6 | 0.8 |
| Other | 12 | 10.8 | 0.7 |
| Pain | 8 | 7.2 | 0.5 |
| Unrelated to mastectomy | 7 | 6.3 | 0.4 |
| Infection | 6 | 5.4 | 0.4 |
| Syncope | 4 | 3.6 | 0.2 |
| Nausea/vomiting | 3 | 2.7 | 0.2 |
RTC, return to care; SHR, surgical home recovery.
Discussion
Our study found that adopting enhanced recovery principles and changing a culture of care can allow for SHR after mastectomy for a majority of patients. Of the 1,689 patients who underwent outpatient mastectomy (OM), 1,578 (93%) had a successful home recovery without RTC. The mean LOS for admitted patients was 28 hours (1.2 days), saving 2,027 inpatient days during 22 months (1,689 outpatients times 1.2 days) within our integrated health delivery system. Ackerman and colleagues16 reported that even a modest decrease in LOS after adopting ERAS protocols for mastectomy (mean LOS 1.44 days with ERAS vs 1.19 days without ERAS) translated to 100 hospital bed days that could be reallocated and led to $2.1 million additional revenue at a large comprehensive cancer center. As a high-volume procedure, SHR for mastectomy is a key target in delivering value-based care.
A primary concern about outpatient mastectomy is safety. Other studies have suggested that same-day discharge can compromise the quality of care, and have called for detailed studies examining patient-level quality metrics in outpatient mastectomy.17 The strength of this study is the inclusion of patients undergoing reconstruction, bilateral operations, neoadjuvant chemotherapy, and detailed information about perioperative pain management. The adoption of home recovery did not compromise quality of care, with only 1.6% of SHR patients requiring a reoperation. Our reoperation rate is lower than the published 5.4% reoperation rate for mastectomy in a NSQIP review, demonstrating that same-day discharge for mastectomy can be performed safely.18
After analyzing patient-level and operative factors as well as perioperative pain control, only 2 variables increased the likelihood for RTC. African American patients were more than 2.5 times as likely to RTC (OR 2.6; 95% CI, 1.38 to 4.91). ASA class 3 to 4 also doubled the odds of an unplanned RTC (OR 2.01; 95% CI, 1.29 to 3.14). Another study evaluating racial disparities in OM found that after adjustment for multiple confounders, Black patients were less likely to undergo OM (OR 0.86; 95% CI, 0.80 to 0.93) compared with White patients.19 Although race/ethnicity was not a significant predictors of OM in our study, it was found to be significant in regard to unplanned RTC. This has been seen previously in other major elective operations, with Black race associated with an increased likelihood for readmission, with OR 1.13 after colectomy and 1.44 after gastric bypass.20 There is established literature demonstrating that there can be a racial bias in perception of pain.21 However, in a chart review of the 111 patients who had an unplanned RTC in our cohort, only 8 patients presented with pain as the chief symptom. With these few patients, we were unable to establish any meaningful relationship between race/ethnicity and adequate pain control.
Compromised access to immediate reconstruction is another concern about outpatient mastectomies. In the US, the Women’s Health and Cancer Rights Act of 1998 established postmastectomy reconstruction as a federally mandated benefit, and immediate reconstruction is often considered a surrogate for surgical quality. Bian and colleagues22 reviewed the Surveillance, Epidemiology, and End Result-Medicare data from 1998 to 2002, with 21% of the mastectomies performed outpatient and only 4% of these patients underwent postmastectomy reconstruction.22 In a more modern review of the California Office of Statewide Health Planning and Development data, the rate of outpatient mastectomy ranged from 20.4% to 23.9% from 2006 to 2009, with the rate of outpatient immediate reconstruction rising from 7.7% to 10.3% (overall rate 9.1%).9 In our organization, there was no change in the overall reconstruction rate as mastectomy was transitioned to an outpatient procedure. Our study presents the largest series of outpatient bilateral mastectomies (n = 393 [23.2%]), mastectomy with immediate reconstruction (n = 517 [30.3%]), and bilateral mastectomies with immediate reconstruction (n = 190 [11.2%]). Although both bilateral operation (OR 0.70; 95% CI, 0.54 to 0.91) and immediate reconstruction (OR 0.52; 95% CI, 0.39 to 0.70) decrease the likelihood of SHR, neither of these operative factors significantly increased the likelihood of an unplanned RTC.
The most significant predictor of SHR is undergoing operation by a high-volume breast surgeon (OR 2.12; 95% CI, 1.42 to 3.18). High-volume breast surgeons were defined as those performing more than 50 breast operations annually within our integrated health delivery system. This definition has been used previously in a study by Morrow and colleagues,23 and correlated with an increased adherence to evidence-based practice. Breast surgeons from all 21 medical centers in our health system meet quarterly to share best practices. We attribute a large part of the successful, rapid implementation of the SHR initiative to the communication among breast leaders, adoption of new practices, and willingness to disseminate this information within their respective facilities.
Other predictors of successful SHR included multimodal pain management. Multimodal anesthesia is one of the overarching principles of ERAS, which has been shown to decrease LOS across many surgical subspecialties.13 , 24 , 25 Smaller studies have found that applying ERAS principles to patients undergoing breast operations enabled same-day discharge without any effect on complication rates.26 , 27 Dumestre and colleagues10 reported that by applying ERAS principles to patients undergoing breast operations, patients who underwent alloplastic breast reconstruction followed by an ERAS protocol (n = 78, mean LOS 0.3 nights) vs a Traditional Recovery after Surgery (TRAS) protocol (n = 78, mean LOS 1.45 nights) found no difference in 30-day emergency department visit rate (8% ERAS vs 14% TRAS; p = 0.20), readmissions (8% ERAS vs 3.8% TRAS; p = 0.30), or rate of hematoma (0.7% ERAS vs 0% TRAS; p = 0.35), concluding that ERAS is a safe approach. IV acetaminophen and receipt of perioperative opiates were associated with 59% and 47% increased odds of outpatient mastectomy, respectively. IV acetaminophen also significantly reduced the likelihood of having an unplanned RTC (OR 0.56; 95% CI, 0.35 to 0.88). Unexpectedly, we found that NSAIDs and gabapentin decreased the likelihood of home recovery. One explanation is that the standard acetaminophen and opiates were not sufficient and, therefore, the need to add NSAIDs and gabapentin is a surrogate for overall inadequate pain control necessitating inpatient admission. The overall low use of NSAIDs, seen in 13% of the outpatient mastectomies, is likely due to the concern for increased risk of bleeding and might reflect the difficulty of the operation.
There were limitations in regard to pain management data. Only aggregate data on anesthesia placed locoregional blocks were included, such as pectoralis nerve plane (PECS I and PECS II), paravertebral, and erector spinae locations. Any intraoperative surgeon-performed block was not captured. We also did not differentiate between the type of local anesthetic used, such as liposomal bupivacaine vs standard bupivacaine. In addition, opioid use postoperatively indicated that a prescription was filled, but the exact morphine equivalent that was taken by the patient is unknown in this study. For this reason, to obtain a global assessment of patients’ perioperative pain control, we used a delta pain score. Patients undergoing SHR had a lower delta pain score, translating into better pain control (–1.8 SHR vs –0.9 inpatient; p > 0.01). The delta pain score was not a significant predictor of same-day discharge or RTC.
Another limitation of this study was the lack of patient reported outcomes. However, multiple earlier studies have shown that there is increased patient satisfaction with SHR.2 , 28 , 29 Dumestre and colleagues30 compared the following cohorts based on style of recovery: traditional (n = 29), transition (n = 11), and ERAS (n = 29); ERAS patients had less severe pain (p = 0.02) and nausea (p = 0.01), enjoyed their food more (p = 0.0002), and felt more rested (p = 0.02). A recent Canadian study by Keehn and colleagues31 examined patient-reported outcomes after adoption of outpatient mastectomy in the Alberta province. They found that 90% of participants felt “excellent or good” with plan to go home, 90% felt “excellent or good” to take care of themselves once home, and 87% felt “excellent or good” with how to take care of their drain. SHR permits patients to recuperate in a familiar environment, leading to better physical and psychological recovery that emphasizes patient comfort, control, and independence.
Conclusions
This study was initiated before the COVID-19 pandemic, however, the results within are timely and relevant to the current needs of our healthcare system. Our earlier study found that outpatient mastectomy is safe and feasible, with no increase in unplanned RTC, as mastectomy has been transitioned to a predominantly outpatient procedure in a large integrated health system. The current study provides data guiding surgeons in patient selection for SHR, which aligns with the COVID-19 Pandemic Breast Cancer Consortium priority to decrease LOS,32 , 33 including showing that patients undergoing immediate reconstruction or bilateral mastectomy can be discharged home the same day without an increased likelihood of RTC. High-volume breast surgeons and multimodal pain therapy increase the odds of same-day discharge. ASA class 3 to 4 and African American race increase likelihood of RTC, identifying potentially vulnerable populations that can benefit from additional outreach.
Author Contributions
Study conception and design: Vuong, Chang, Mentakis, Shim, Schmittdiel, Kuehner
Acquisition of data: Vuong, Dusendang, Kuehner
Analysis and interpretation of data: Vuong, Dusendang, Schmittdiel, Kuehner
Drafting of manuscript: Vuong, Dusendang, Chang, Schmittdiel, Kuehner
Critical revision: Vuong, Dusendang, Chang, Mentakis, Shim, Schmittdiel, Kuehner
Footnotes
Disclosure Information:Authors have nothing to disclose.
References
- 1.Shahbazi S., Woods S.J. Influence of physician, patient, and health care system characteristics on the use of outpatient mastectomy. Am J Surg. 2016;211:802–809. doi: 10.1016/j.amjsurg.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Bonnema J., van Wersch A.M., van Geel A.N. Medical and psychosocial effects of early discharge after surgery for breast cancer: randomised trial. BMJ. 1998;316(7140):1267–1271. doi: 10.1136/bmj.316.7140.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamtani A., Morrow M. Why Are there so many mastectomies in the United States? Annu Rev Med. 2017;68:229–241. doi: 10.1146/annurev-med-043015-075227. [DOI] [PubMed] [Google Scholar]
- 4.Steiner C.A., Weiss A.J., Barrett M.L. AHRQ; Rockville, MD: 2016. Trends in Bilateral and Unilateral Mastectomies in Hospital Inpatient and Ambulatory Settings, 2005–2013: Statistical Brief #201. [PubMed] [Google Scholar]
- 5.Cordeiro E., Jackson T., Cil T. Same-day major breast cancer surgery is safe: an analysis of short-term outcomes using NSQIP data. Ann Surg Oncol. 2016;23:2480–2486. doi: 10.1245/s10434-016-5128-0. [DOI] [PubMed] [Google Scholar]
- 6.Marla S., Stallard S. Systematic review of day surgery for breast cancer. Int J Surg. 2009;7:318–323. doi: 10.1016/j.ijsu.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Goodman A.A., Mendez A.L. Definitive surgery for breast cancer performed on an outpatient basis. Arch Surg. 1993;128:1149–1152. doi: 10.1001/archsurg.1993.01420220069009. [DOI] [PubMed] [Google Scholar]
- 8.Simpson S.A., Ying B.L., Ross L.A. Incidence of complications in outpatient mastectomy with immediate reconstruction. J Am Coll Surg. 2007;205:463–467. doi: 10.1016/j.jamcollsurg.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Kruper L., Xu X.X., Henderson K. Utilization of mastectomy and reconstruction in the outpatient setting. Ann Surg Oncol. 2013;20:828–835. doi: 10.1245/s10434-012-2661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumestre D.O., Redwood J., Webb C.E., Temple-Oberle C. Enhanced Recovery after Surgery (ERAS) protocol enables safe same-day discharge after alloplastic breast reconstruction. Plast Surg. 2017;25:249–254. doi: 10.1177/2292550317728036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuong B., Graff-Baker A.N., Yanagisawa M. Implementation of a post-mastectomy home recovery program in a large, integrated health care delivery system. Ann Surg Oncol. 2019;26:3178–3184. doi: 10.1245/s10434-019-07551-0. [DOI] [PubMed] [Google Scholar]
- 12.Mayhew D., Mendonca V., Murthy B.V.S. A review of ASA physical status—historical perspectives and modern developments. Anaesthesia. 2019;74:373–379. doi: 10.1111/anae.14569. [DOI] [PubMed] [Google Scholar]
- 13.Hedderson M., Lee D., Hunt E. Enhanced Recovery after Surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511–519. doi: 10.1097/AOG.0000000000003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickless A., Voysey M., Geddes J. Mixed effects approach to the analysis of the stepped wedge cluster randomised trial—investigating the confounding effect of time through simulation. PLoS One. 2018;13(12):1–22. doi: 10.1371/journal.pone.0208876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Protection of Human Subjects, Definitions. 45 CFR §46.102(f) (1991).
- 16.Ackerman R.S., Hirschi M., Alford B. Enhanced REVENUE after surgery? A cost-standardized enhanced recovery pathway for mastectomy decreases length of stay. World J Surg. 2019;43:839–845. doi: 10.1007/s00268-018-4850-0. [DOI] [PubMed] [Google Scholar]
- 17.Case C., Steiner C. Outpatient mastectomy: clinical, payer, and geographic influences. HSR Heal Serv Res. 2001;36:869–884. [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hilli Z., Thomsen K.M., Habermann E.B. Reoperation for complications after lumpectomy and mastectomy for breast cancer from the 2012 National Surgical Quality Improvement Program (ACS-NSQIP) Ann Surg Oncol. 2015;22:459–469. doi: 10.1245/s10434-015-4741-7. [DOI] [PubMed] [Google Scholar]
- 19.Salasky V., Yang R.L., Datta J. Racial disparities in the use of outpatient mastectomy. J Surg Res. 2014;186:16–22. doi: 10.1016/j.jss.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 20.Wilson G.C., Cutler Quillin R., Sutton J.M. Factors related to readmission after major elective surgery. Dig Dis Sci. 2015;60:47–53. doi: 10.1007/s10620-014-3306-0. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman K.M., Trawalter S., Axt J.R., Oliver M.N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296–4301. doi: 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian J., Krontiras H., Allison J. Outpatient mastectomy and breast reconstructive surgery. Ann Surg Oncol. 2008;15:1032–1039. doi: 10.1245/s10434-007-9762-4. [DOI] [PubMed] [Google Scholar]
- 23.Morrow M., Jagsi R., McLeod M.C. Surgeon attitudes toward the omission of axillary dissection in early breast cancer. JAMA Oncol. 2018;4:1511–1516. doi: 10.1001/jamaoncol.2018.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljungqvist O., Scott M., Fearon K.C. Enhanced Recovery after Surgery: a review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 25.Yanik J.M., Bedard N.A., Hanley J.M. Rapid recovery total joint arthroplasty is safe, efficient, and cost-effective in the Veterans Administration setting. J Arthroplasty. 2018;33:3138–3142. doi: 10.1016/j.arth.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Tan L.R., Guenther J.M. Outpatient definitive breast cancer surgery. Am Surg. 1997;63:865–867. [PubMed] [Google Scholar]
- 27.McManus S.A., Topp D.A., Hopkins C. Advantages of outpatient breast surgery. Am Surg. 1994;60:967–970. [PubMed] [Google Scholar]
- 28.Kambouris A. Physical, psychological, and economic advantages of accelerated discharge after surgical treatment for breast cancer. Am Surg. 1996;62:123–127. [PubMed] [Google Scholar]
- 29.Margolese R.G., Lasry J.C. Ambulatory surgery for breast cancer patients. Ann Surg Oncol. 2000;7:181–187. doi: 10.1007/BF02523651. [DOI] [PubMed] [Google Scholar]
- 30.Dumestre D.O., Webb C.E., Temple-Oberle C. Improved recovery experience achieved for women undergoing implant-based breast reconstruction using an Enhanced Recovery after Surgery model. Plast Reconstr Surg. 2017;139:550–559. doi: 10.1097/PRS.0000000000003056. [DOI] [PubMed] [Google Scholar]
- 31.Keehn A.R., Olson D.W., Dort J.C. Same-day surgery for mastectomy patients in Alberta: a perioperative care pathway and quality improvement initiative. Ann Surg Oncol. 2019;26:3354–3360. doi: 10.1245/s10434-019-07568-5. [DOI] [PubMed] [Google Scholar]
- 32.American College of Surgeons COVID-19 Pandemic Breast Cancer Consortium’s considerations for re-entry. https://www.facs.org/-/media/files/covid19/covid_breast_consortium_reentry.ashx Available at: Published 2020. Accessed June 13, 2020.
- 33.American College of Surgeons American College of Surgeons local resumption of elective surgery guidance. https://www.facs.org/covid-19/clinical-guidance/resuming-elective-surgery Available at: Published 2020. Accessed June 12, 2020.