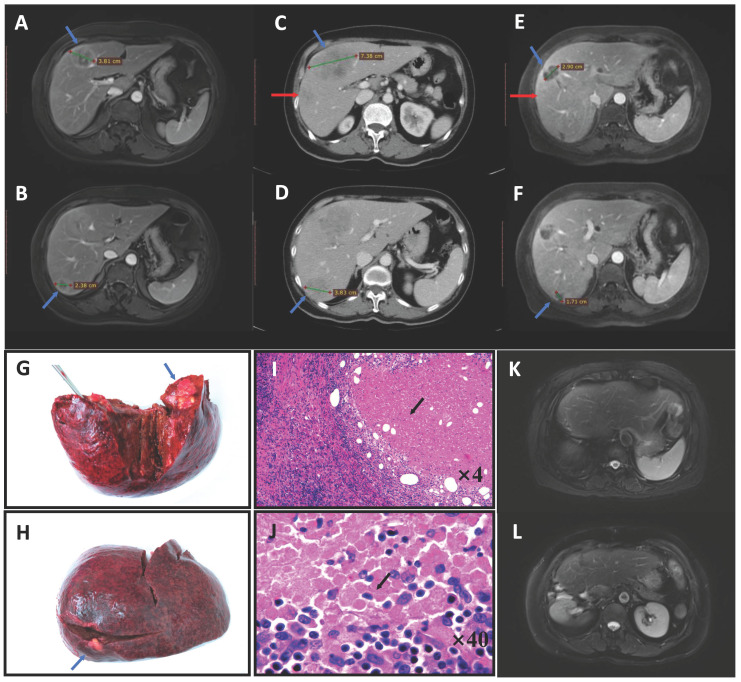
Figure 1.
Pathological complete response (pCR) in a patient with colorectal liver metastases who received pembrolizumab. A 71-year-old woman was diagnosed with simultaneous liver metastases from ascending colon cancer in our center in August 2017. Gene testing showed the tumor was of the RAS/BRAF wild-type, MSI-H. MRI indicated two lesions (blue arrow) that were resectable (A, B). After two cycles of pembrolizumab, the tumor progressed and a new lesion appeared on CT (red arrow) (C, D). We administered two cycles of HAI with systematic oxaliplatin combined with two cycles of pembrolizumab, after which the tumor had significantly shrunk on MRI (E, F). Right hepatic lobectomy and simultaneous right hemicolectomy were then performed (liver specimen G, H). Pathologic examination found no tumor cells in either the liver or primary tumor tissue, with these regions having been replaced by tumor necrosis, fibro-collagenous proliferation and inflammatory cells (hematoxylin-eosin-stained, I: ×4 tumor-normal liver interface, J: ×40 tumor tissue), except for in one positive lymph node (No. 14v) (ypT0N1aM0). The patient was still disease-free after 2 years on MRI (K, L) and had all the four factors predictive of pCR in this study (liver metastases <3 cm, preoperative carcinoembryonic antigen level ≤20 ng/mL, primary T stage 1-2, and a right-sided primary tumor). Abbreviations: MSI-H, microsatellite instability-high; MRI, magnetic resonance imaging; CT, computed tomography; HAI, hepatic arterial infusion