Fig. 5.
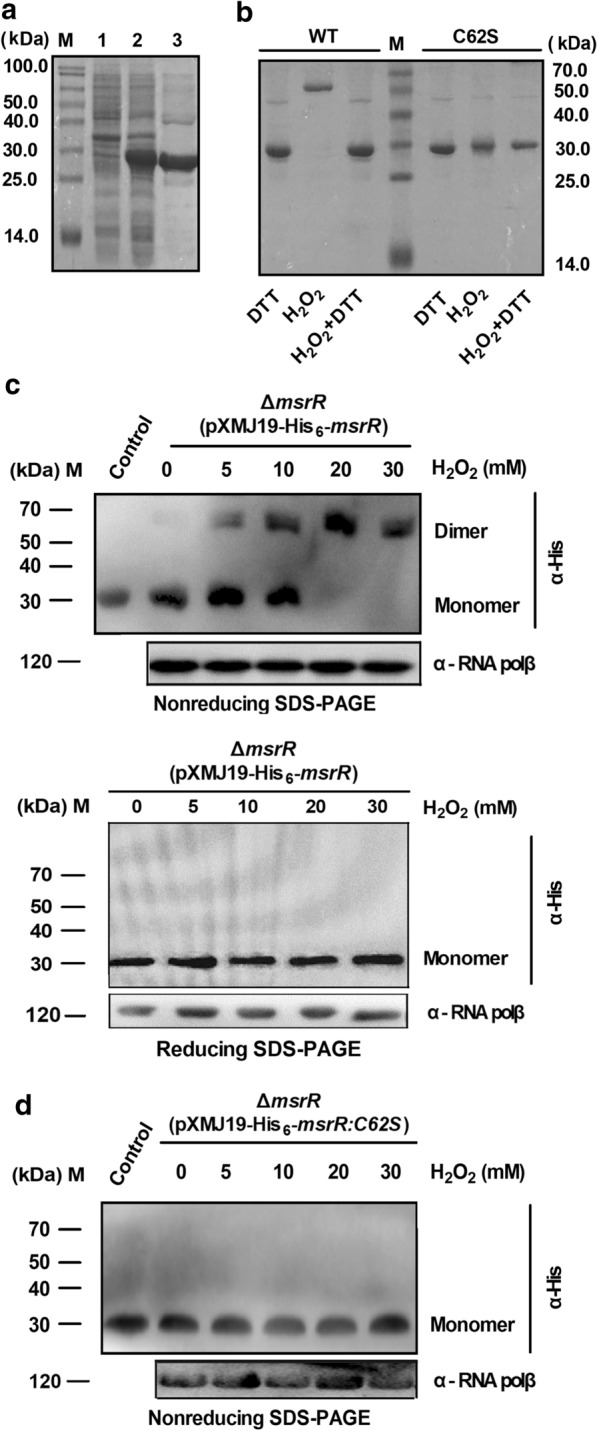
Redox response of MsrR in vitro and in vivo. a Nonreducing SDS-PAGE analysis of proteins expressed in E. coli containing pET28a-msrR plasmid. M, broad-range protein marker; lane 1, crude extract (5 μg) without IPTG induction; lane 2, crude extract (5 μg) with induction; lane 3 purified His6-MsrR protein. b Redox response of MsrR and its variant detected by nonreducing SDS-PAGE. 15 μM proteins treated with 50 mM DTT were further incubated with or without 50 μM H2O2, or 50 μM H2O2 and 50 mM DTT, and then samples were separated by 15% nonreducing SDS-PAGE. c, d Oxidative stress-dependent structural changes of relevant MsrR in vivo. Proteins extracted from cells exposed to different concentrations of H2O2 for 30 min were resolved on nonreducing or reducing SDS-PAGE, and analyzed with Western blotting by using the anti-His antibody. RNA polymerase β (RNA polβ) was used as a loading control. Similar results were obtained in three independent experiments, and data show one representative experiment done in triplicate
