Abstract
Understanding the emergence of collective behaviour has long been a key research focus in the natural sciences. Besides the fundamental role of social interaction rules, a combination of theoretical and empirical work indicates individual speed may be a key process that drives the collective behaviour of animal groups. Socially induced changes in speed by interacting animals make it difficult to isolate the effects of individual speed on group-level behaviours. Here, we tackled this issue by pairing guppies with a biomimetic robot. We used a closed-loop tracking and feedback system to let a robotic fish naturally interact with a live partner in real time, and programmed it to strongly copy and follow its partner's movements while lacking any preferred movement speed or directionality of its own. We show that individual differences in guppies' movement speed were highly repeatable and in turn shaped key collective patterns: a higher individual speed resulted in stronger leadership, lower cohesion, higher alignment and better temporal coordination of the pairs. By combining the strengths of individual-based models and observational work with state-of-the-art robotics, we provide novel evidence that individual speed is a key, fundamental process in the emergence of collective behaviour.
Keywords: speed, collective behaviour, guppy, individual differences, robot, social
1. Introduction
Understanding the emergence of collective behavioural patterns has long been a key research focus in the natural sciences. Considerable theoretical and experimental work has accumulated that describes how complex collective patterns may arise via relatively simple mechanisms [1,2], including the role of phenotypic heterogeneity within and among groups [3]. A fundamental insight is that social interaction rules at the individual level—such as avoiding others that are too near and approaching those far away—can explain the large-scale cohesion, coordination and decision-making of animal groups [2,4].
Most animals control their motion by modulating their speed and turning, and this speed regulation has been shown to be crucial for the attraction and avoidance behaviour when animals group and interact [5–7]. Hence, individual speed may be an additional fundamental factor that underlies the emergence of the global properties of groups. Indeed, both short-term changes and heterogeneity in speed have been linked to a range of group-level properties, such as group cohesion, structure, shape, coordination, and leadership by theoretical analyses [8], computer simulations [1,4,9] and empirical work [6,10–14]. Importantly, grouping individuals may differ in their preferred and optimal movement speeds yet must also coordinate and adjust their behaviour to successfully group together [3,15]. Such socially induced changes in speed by individuals interacting with one another make it difficult to empirically isolate the effects of individual speed for group-level properties. And while with agent-based simulations one can separately model such effects and thereby make important predictions for collective behaviour, they are no substitute for empirical data of real animal groups [2].
Due to recent advances in the field of robotics, robotic individuals now not only realistically look and behave like conspecifics, but also interact with live animals in a natural way [16–19]. This sets the stage for manipulative experiments where part of the group members can be controlled and programmed with theoretical models under investigation. Here, we present results from such an experiment using live guppies (Poecilia reticulata) swimming with an interactive biomimetic fish-like robot (robofish) to examine the role of fish's individual movement speed on collective behavioural patterns. We combined high-definition video tracking and a closed-loop feedback system that used interaction rules from well-known agent-based models [4] to steer the robot interactively in real time [20,21]. We programmed the robot to always follow its partner and copy its behaviour, while excluding any preferred swimming speed or directionality, thus enabling us to determine how individual differences in the guppies' movement speed alone determined group-level properties, in terms of leadership, cohesion, alignment and temporal coordination.
2. Methods
We used laboratory-reared descendants of wild-caught Trinidadian guppies that were housed in large, randomly out-bred mixed-sex stock tanks under controlled laboratory conditions (12 h : 12 h light : dark; 26°C). We randomly selected 20 naive adult females (standard length ‘BL’: 31.7 ± 0.8 mm) and moved them to individual holding tanks (40 × 20 × 25 cm). The following week, we tested fish first without robofish to assess their preferred movement speed (week 2) and then twice with the robofish (week 3; trial 2 five days later). Throughout, fish were fed twice daily ad libitum with TetraMin flake food.
The test arena consisted of a large white glass tank (88 cm × 88 cm, water height 7.5 cm) that was illuminated from above and enclosed to minimize potential external disturbances. Fish were moved from their individual holding compartment to the experimental tank where they were allowed to acclimatize for 1 min in an opaque PVC cylinder in the corner of the tank. After the cylinder was raised, the fish filmed from above for 10 min, and its movements automatically tracked at 30 fps using BioTracker [22]. For the trials with the robotic fish, we used a three-dimensional-printed fish replica resembling a female guppy that was connected via magnets to a two-wheeled robot below the tank (see electronic supplementary material, figure S1 and for details [21]). The robot was controlled via a closed-loop system whereby the movements of the fish were tracked and fed-back to the robot control. The robot unit then adjusted its position and orientation in real time (i.e. with 30 hertz) to result in natural response times. Robofish was circling in front of the acclimatization cylinder and as soon as the guppy was released from the cylinder started its interactive behaviour.
Robofish's interactive behaviour was based on the zonal model [4] and allowed the robot to copy the live fish's motions and follow at a similar speed without a preferred speed or directional preference of their own (figure 1 and electronic supplementary material, figure S2). We programmed robofish to orientate towards the live fish's position and stay at a distance between 10–15 cm away (∼4 BL, ‘optimal distance zone’), reflecting spacing observed in wild guppies [23]. This resulted in robofish following at the instantaneous speed exhibited by the live fish while it was in this optimal distance zone. Robofish gradually decreased or increased its speed when the focal fish got into the graduation zone (3–10 cm) or beyond the optimal distance zone, respectively. If the focal fish was at a distance less than 3 cm away, robofish stopped moving forward but kept turning at its location to focus on the live fish's position. The maximum speed and acceleration of robofish were set to reflect that observed for the guppies when alone (25 cm s−1 and 2.5 cm s−2 respectively, see electronic supplementary material, figure S3), with its maximum turning rate being greater than 360°/s.
Figure 1.
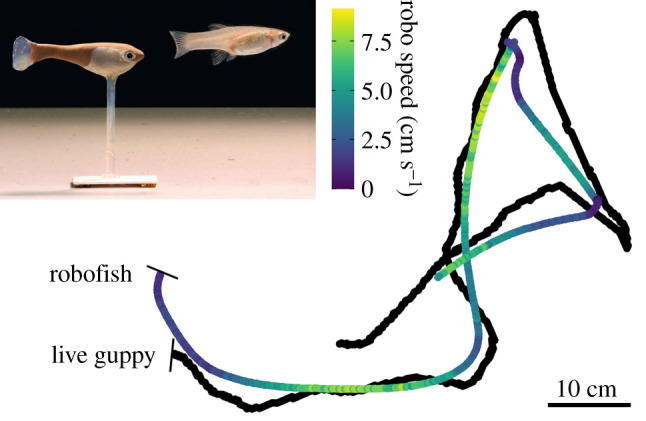
Tracking data (approx. 1 min) of a randomly selected representative pair, with the speed of the robofish coloured blue (low) to yellow (high), showing how it followed the position and movements of its partner by natural changes in speed (see further electronic supplementary material, figure S2). Inset shows a photo of robofish following a guppy. Data were subsetted to a region where the guppy made a number of turns and changes in speed.
Tracking data were checked for errors, processed to correct for missing frames, and converted to millimetres. Subsequently, based on the centroid of each individual (focal and robotic), we calculated speed and heading as well as inter-individual distance. For each trial, we computed the fish's median speed, the median inter-individual distance, median difference in heading angle, and proportion of time the focal fish was in front (when moving greater than 0.5 cm s−1; ‘leadership’). In addition, we computed their coordination by running temporal correlations of both individuals' change in speed and heading, with a higher correlation indicating movement changes were better copied between them. We used a linear-mixed modelling approach to investigate relationships in the individual- and group-level metrics as well as repeatability in behaviour. Further details of our methods and statistics can be found in the electronic supplementary material.
3. Results
There were large and significant among-individual differences in guppies’ movement speed across the two robofish trials (R = 0.70, 95% confidence interval (CI) = 0.38–0.88), which correlated well with their movement speed when tested alone (χ2 = 9.70, p = 0.002, electronic supplementary material, figure S4). Although the majority of fish slowed down when tested with robofish (30/38 trials), there was large among-individual variation in fish's speed relative to that expressed in the solo assay (0.62 ± 0.06, 95% CI = 0.15–1.29; a value of 1 would indicate no change in speed), which was itself not linked to their solo speed (χ2 = 0.155, p = 0.212, R2mar = 0.06).
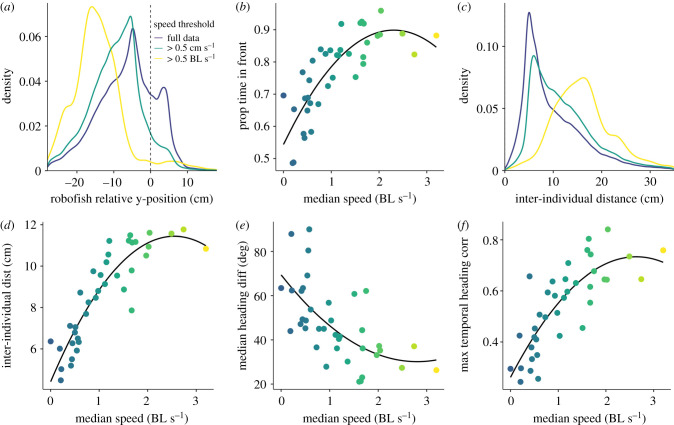
Robofish conformed extremely well to the speed of the guppy partner (χ2 = 87.04, p < 0.001, electronic supplementary material, figure S5) and consistently exhibited a slightly slower speed (speed difference: −0.58 ± 0.07 cm s−1). As a consequence, robofish primarily occupied the following position (38/38 trials, electronic supplementary material, figure S6), an effect that strongly increased with the speed of the focal fish (χ2 = 40.77, p < 0.001, ), with the fastest guppies leading more than 90% of the time (figure 2a,b).
Figure 2.
(a,c) Density plot of robofish's relative y-position and distance to its live partner, and (b,d,e,f) scatterplots of fish's median speed in relation to the leadership, cohesion, alignment and temporal coordination of the pair. Plots a and c show both the full data (blue line), the data was subsetted to where the focal fish moved at >0.5 cm/s (green line), and at >0.5 BL/s (yellow line). Colour scale indicates speed (blue = low; yellow = high) and solid lines show the polynomial functions fitted in our models.
Robofish was able to maintain good cohesion and alignment with its live partner, and naturally copied its changes in speed and heading (median max correlation coefficients: 0.51 and 0.58, electronic supplementary material, figure S7). As for leadership, we found that these group-level outcomes were very well explained by the individual speed of the guppy. Pairs in which the guppy had a high median speed were considerably less cohesive (χ2 = 60.04, p < 0.001, ), more aligned (χ2 = 13.66, p < 0.001, ) and more coordinated (χ2 = 41.33, p < 0.001, ) than pairs with a much slower guppy (figure 2c–f).
4. Discussion
Live guppies paired with an extremely social robotic fish showed large and repeatable individual differences in movement speeds that in turn strongly explained leadership, group cohesion, alignment and movement coordination. By testing all fish with the same robot using identical interaction rules and lacked any preferred movement speed and directionality, these results provide novel experimental evidence that suggest that individual speed is a fundamental factor in the emergence of collective behavioural patterns, in line with existing theoretical and empirical work [4,8,12–14]. As individual differences in speed are associated with a broad range of phenotypic traits observed among grouping animals, this may also help provide a mechanistic explanation for the effect of phenotypic heterogeneity of group-level patterns [3], as has for example been shown for size, hunger and parasitism [24,25].
We observed a very strong, positive link between speed and leadership, both in terms of clearer front-back positioning the faster fish were moving, as well as fish being overall more in front the higher their median movement speed. This result is in line with predictions from model simulations [4,12] and previous empirical work [11,12]. By testing fish with an extremely social partner that always tried to follow, we found that at higher speeds fish led almost all the time. This shows how leaders depend on the responsiveness of their followers in order to express their own preference (see also [26]) and more generally highlights how both individual speeds and high levels of social responsiveness are important for the collective performance of groups [27]. At lower speeds, leadership differences were not as apparent, potentially because individuals have greater turning freedom at lower speeds [8]. The absolute speed at which a group is moving may therefore be just as important as relative differences in speed between individuals in shaping group structure. Our experiments show that the observed effects of speed emerge from the self-organized dynamics of the coupled leader–follower system, i.e. that it does not require any diverging preferences of the follower. An analogy can be drawn to a physical system, where the follower can be viewed as an ‘active particle’ strongly coupled to the leader through a spring-like social force with friction.
Pairs consisting of a guppy with a high median speed were considerably less cohesive than those with low median speeds, in line with previous work on schooling sticklebacks [12]. To avoid collisions, grouping animals may actively increase their distance when moving at higher speeds. However, robofish was not programmed with such a rule, suggesting that the observed positive link between cohesion and speed is due to speed mediating the use of social interaction rules: faster individuals moving farther before they can change their position in response to their group mates. This suggests that a shift in interaction rules, such as via changes in the environment, may alter the relationship between group speed and cohesion (see e.g. [28,29]). Individual speed also strongly drove the alignment and temporal coordination of the pairs, in line with previous empirical studies that found fast-moving groups tend to be polarized and slow-moving groups to be disordered [12,13,30]. Since in our study the robotic partner completely lacked any alignment rules, our findings provide novel empirical evidence that individual speed is a key factor facilitating group alignment and coordination. Although speed-mediated changes in local interaction rules may help to explain these effects [6,10], groups may be more likely to become disordered at lower speeds because of larger potential angular fluctuations at lower speeds, as is predicted from the theoretical analysis [8]. Given that rates of information flow have been shown to be higher in faster-moving groups [13], our finding that faster groups showed better coordination of movement changes can therefore be explained by the higher (local) order that arises with higher individual speeds.
The large individual differences in movement speed during the robofish trials were highly repeatable (R = 0.70), as compared to the average repeatability of 0.37 reported by a large meta-analysis [31]. As robofish always copied its live partner's speed and movements, and always used the same interaction rules, the large variability in movement speed among the pairs must be attributable to the speed of the live fish, which was in turn well explained by fish's solo speed. Interestingly however, considerable speed variation among the fish remained. The reduction in movement speed between the solo and robofish trials therefore likely reflects socially mediated changes, with guppies that slowed down more being more socially responsive and/or less inclined to lead. This corroborates previous observational work that found live fish pairs moved faster when led by fish that were less socially plastic in their speeding changes [7]. Future work is needed to properly determine to what extent this behavioural variation in ‘social speed’ indicates true individual differences in social responsiveness.
In summary, by closed-loop experiments of live guppies swimming with a biomimetic robot that always followed and naturally copied its partners' movements, we bridged the reality gap between computer simulations and real-world observations and provide novel evidence that individual speed is a fundamental factor for the emergence of collective behaviour. By programming the robotic fish without any of its own movement preferences, we had the unique opportunity to investigate how individual behaviour leads to group-level patterns without the potential influence of individual heterogeneity in group mates. Exciting interdisciplinary work lies ahead to further investigate the role that individuals play in animal groups and how that depends on the social feedback among heterogeneous group members.
Supplementary Material
Supplementary Material
Acknowledgements
We thank two anonymous reviewers for their helpful feedback. We are also very grateful to Hauke Mönck and Hai Nguyen for help with programming the robot.
Ethics
All reported experiments comply with the current German law approved by LaGeSo (G0117/16 to D.B.).
Data accessibility
Data accompanying this paper can be found in the electronic supplementary material.
Authors' contributions
J.W.J., N.W. and D.B. designed the study; T.L., D.B., P.R. and J.K. developed robofish; N.W. and D.B. conducted the experiment with help from J.W.J. J.W.J. performed the analysis; J.W.J. and D.B. wrote the manuscript with feedback from all other authors. All authors agree accountability for the content in this paper and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge support from the Alexander von Humboldt-Stiftung (postdoctoral fellowship to J.W.J.), the Zukunftskolleg, University of Konstanz (postdoctoral fellowship to J.W.J.), the German Research Foundation (BI 1828/2-1, LA 3534/1-1, RO 4766/2-1) and Germany's Excellence Strategy (EXC 2002/1 ‘Science of Intelligence’, project number 390523135).
References
- 1.Couzin ID, Krause J. 2003. Self-organization and collective behavior in vertebrates. Adv. Study Behav. 32, 1–75. ( 10.1016/S0065-3454(03)01001-5) [DOI] [Google Scholar]
- 2.Herbert-Read JE. 2016. Understanding how animal groups achieve coordinated movement. J. Exp. Biol. 219, 2971–2983. ( 10.1242/jeb.129411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolles JW, King AJ, Killen SS. 2020. The role of individual heterogeneity in collective animal behaviour. Trends Ecol. Evol. 35, 278–291. ( 10.1016/j.tree.2019.11.001) [DOI] [PubMed] [Google Scholar]
- 4.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11. ( 10.1006/yjtbi.3065) [DOI] [PubMed] [Google Scholar]
- 5.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJT, Ward AJW. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. 108, 18 726–18 731. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz Y, Tunstrøm K, Ioannou CC, Huepe C, Couzin ID. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. 108, 18 720–18 725. ( 10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaerf TM, Herbert-Read JE, Myerscough MR, Sumpter DJT, Ward AJW. 2016. Identifying differences in the rules of interaction between individuals in moving animal groups.
- 8.Romanczuk P, Bär M, Ebeling W, Lindner B, Schimansky-Geier L. 2012. Active Brownian particles: from individual to collective stochastic dynamics. Eur. Phys. J. Spec. Top. 202, 1–162. ( 10.1140/epjst/e2012-01529-y) [DOI] [Google Scholar]
- 9.Gueron S, Levin SA, Rubenstein DI. 1996. The dynamics of herds: from individuals to aggregations. J. Theor. Biol. 182, 85–98. ( 10.1006/jtbi.1996.0144) [DOI] [Google Scholar]
- 10.Tunstrøm K, Katz Y, Ioannou CC, Huepe C, Lutz MJ, Couzin ID. 2013. Collective states, multistability and transitional behavior in schooling fish. PLoS Comput. Biol. 9, e1002915 ( 10.1371/journal.pcbi.1002915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettit B, Ákos Z, Vicsek T, Biro D. 2015. Speed determines leadership and leadership determines learning during pigeon flocking. Curr. Biol. 25, 3132–3137. ( 10.1016/j.cub.2015.10.044) [DOI] [PubMed] [Google Scholar]
- 12.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27, 2862–2868. ( 10.1016/j.cub.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent MIA, Lukeman R, Lizier JT, Ward AJW. 2019. Speed-mediated properties of schooling. R. Soc. Open Sci. 6, 181482 ( 10.1098/rsos.181482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumpter DJT, Szorkovszky A, Kotrschal A, Kolm N, Herbert-Read JE. 2018. Using activity and sociability to characterize collective motion. Phil. Trans. R. Soc. B 373, 20170015 ( 10.1098/rstb.2017.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert-Read JE, Krause S, Morrell LJ, Schaerf TM, Krause J, Ward AJW. 2012. The role of individuality in collective group movement. Proc. R. Soc. B 280, 20122564 ( 10.1098/rspb.2012.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause J, Winfield AFT, Deneubourg J-L. 2011. Interactive robots in experimental biology. Trends Ecol. Evol. 26, 369–375. ( 10.1016/j.tree.2011.03.015) [DOI] [PubMed] [Google Scholar]
- 17.Romano D, Donati E, Benelli G, Stefanini C. 2019. A review on animal–robot interaction: from bio-hybrid organisms to mixed societies. Biol. Cybern. 113, 201–225. ( 10.1007/s00422-018-0787-5) [DOI] [PubMed] [Google Scholar]
- 18.Datteri E. 2020. Interactive biorobotics. Synthese ( 10.1007/s11229-020-02533-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Lellis P, Cadolini E, Croce A, Yang Y, Di Bernardo M, Porfiri M.. 2020. Model-based feedback control of live zebrafish behavior via interaction with a robotic replica. IEEE Trans. Robot. 36, 28–41. ( 10.1109/TRO.2019.2943066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria JJ, Krause S, Krause J. 2010. Collective behavior in road crossing pedestrians: the role of social information. Behav. Ecol. 21, 1236–1242. ( 10.1093/beheco/arq141) [DOI] [Google Scholar]
- 21.Landgraf T, Bierbach D, Nguyen H, Muggelberg N, Romanczuk P, Krause J. 2016. RoboFish: increased acceptance of interactive robotic fish with realistic eyes and natural motion patterns by live Trinidadian guppies. Bioinspir. Biomim. 11, 015001 ( 10.1088/1748-3190/11/1/015001) [DOI] [PubMed] [Google Scholar]
- 22.Mönck HJ, et al. 2018. BioTracker: an open-source computer vision framework for visual animal tracking. arXiv 1803.07985.
- 23.Herbert-Read JE, et al. 2017. How predation shapes the social interaction rules of shoaling fish. Proc. R. Soc. B 284, 20171126 ( 10.1098/rspb.2017.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolles JW, Mazué GPF, Davidson J, Behrmann-Godel J, Couzin ID. 2020. Schistocephalus parasite infection alters sticklebacks' movement ability and thereby shapes social interactions. Sci. Rep. 10, 12282 ( 10.1038/s41598-020-69057-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause J, Reeves P, Hoare DJ. 1998. Positioning behaviour in roach shoals: the role of body length and nutritional state. Behaviour 135, 1031–1039. ( 10.1163/156853998792913519) [DOI] [Google Scholar]
- 26.Ioannou CC, Singh M, Couzin ID. 2015. Potential leaders trade off goal-oriented and socially oriented behavior in mobile animal groups. Am. Nat. 186, 284–293. ( 10.1086/681988) [DOI] [PubMed] [Google Scholar]
- 27.Wolf M, Krause J. 2014. Why personality differences matter for social functioning and social structure. Trends Ecol. Evol. 29, 306–308. ( 10.1016/j.tree.2014.03.008) [DOI] [PubMed] [Google Scholar]
- 28.Schaerf TM, Dillingham PW, Ward AJW. 2017. The effects of external cues on individual and collective behavior of shoaling fish. Sci. Adv. 3, e1603201 ( 10.1126/sciadv.1603201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolles JW, Laskowski KL, Boogert NJ, Manica A. 2018. Repeatable group differences in the collective behaviour of stickleback shoals across ecological contexts. Proc. R. Soc. B 285, 20172629 ( 10.1098/rspb.2017.2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viscido SV, Parrish JK, Grünbaum D. 2004. Individual behavior and emergent properties of fish schools: a comparison of observation and theory. Mar. Ecol. Prog. Ser. 273, 239–249. ( 10.3354/meps273239) [DOI] [Google Scholar]
- 31.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data accompanying this paper can be found in the electronic supplementary material.