Figure 3.
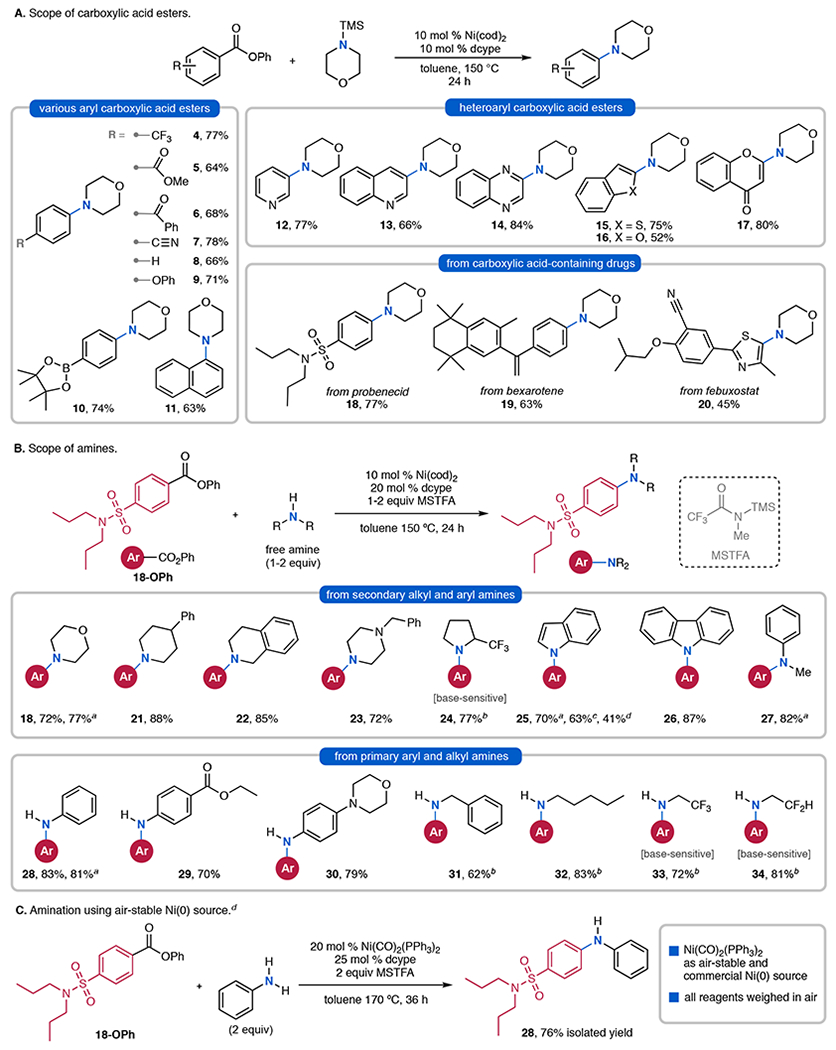
Scope of Ni-catalyzed decarbonylative amination. aUsing TMS–amine. bTMS–amine was generated in situ by premixing the amine with MSTFA. cUsing TES–indole or dTIPPS–indole. dReagents were weighed on benchtop. For additional substrates that were found to be challenging under the optimized conditions, see SI.
