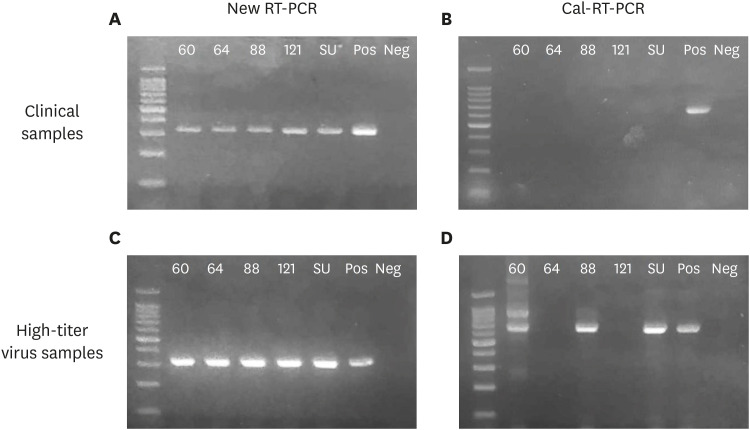
Fig. 3. Detection of FCV by 2 RT-PCR assays. The new RT-PCR assay developed in this study (left panel) and a RT-PCR assay developed by Seal [14] (Cal-RT-PCR, right panel) were performed for all 122 field samples to detect FCV infections. (A) The new RT-PCR assay amplified targeted amplicons (324 bp) from all 5 positive samples identified by virus isolation; however, (B) the old RT-PCR assay did not detect any FCV. (C, D) RT-PCR assays were performed for high virus titer samples of the 5 positive isolates (1010 TCID50). (C) The new RT-PCR assay amplified targeted amplicons (324 bp) from all of the samples, (D) but the old RT-PCR assay amplified the targeted amplicon (673 bp) in only 3 of the 5 positive samples with some non-specific bands. Lane 1, DNA size marker; lanes 2–4, FCV positive samples from healthy stray cats; lanes 5, FCV positive sample from a cat with nasal discharge; lane 6, FCV positive sample from a cat with severe URTD; lanes 7–8, a positive and a negative control, respectively.
FCV, feline calicivirus; RT-PCR, reverse transcription polymerase chain reaction; URTD, upper respiratory tract disease.