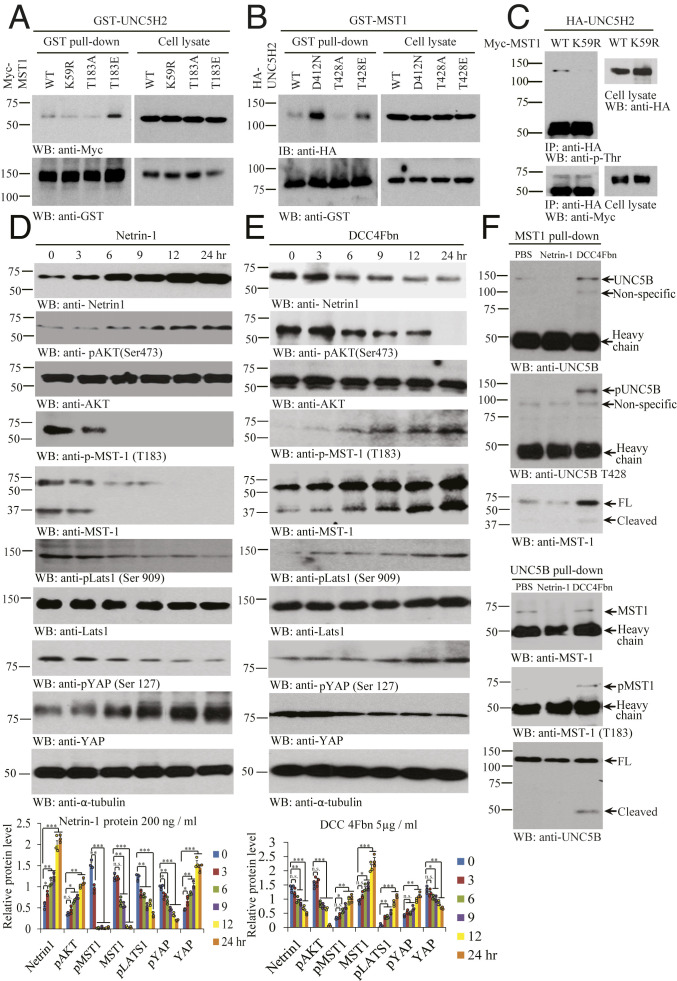
Fig. 3.
NTN1 regulates MST1 and UNC5B phosphorylation and association. (A) MST1 phosphorylation augments the association between UNC5H2 and MST1. GST pulldown of GST-UNC5H2 in Myc-MST1 WT, Myc-MST1 K59R, Myc-MST1 T183A, or Myc-MST1 T183E cotransfected HEK293 cell was confirmed by immunoblotting. (B) Phosphorylated UNC5H2 elevates its interaction with MST1. GST pulldown of GST-MST1 in HA-UNC5H2 WT, HA-UNC5H2 D421N, HA-UNC5H2 T428A, and HA-UNC5H2 T428E cotransfected cells was confirmed by immunoblotting. (C) WT but not KD MST1 (K59R) mutant phosphorylates UNC5H2 in intact cells. HA-UNC5H2 immunocomplex from Myc-MST1 WT and Myc-MST1 K59R transfected cell lysates was detected by immunoblotting with anti-p-Thr or anti-Myc. (D) NTN1 stimulation inhibits Hippo/MST1 signaling pathway. Protein expression levels (NTN1, pAKT [Ser473], AKT, pMST1 [T183], MST1, pLATS1 [Ser909], Lats1, pYAP [Ser127], and YAP) were analyzed by immunoblotting in the NTN1 protein-treated SH-SY5Y cell lysates. (E) NTN1 deprivation activates Hippo/MST1 signaling pathway. Immunoblotting analysis of NTN1, pAKT (Ser473), AKT, pMST1 (T183), MST1, pLATS1 (Ser909), LATS1, pYAP (Ser127), and YAP levels in the DCC-4Fbn−treated SH-SY5Y cell lysates. Error bars represent the mean ± SEM. Statistical significance was determined using a two-way ANOVA followed by post hoc Bonferroni test for multiple group comparison. *P < 0.05; **< 0.01; ***P < 0.001; n.s., not significant. (F) NTN1 deprivation stimulates UNC5B/MST1 association via reciprocal IP. IPs of MST (upper three panels) and UNC5B (lower three panels) from SH-SY5Y cells treated with vehicle, NTN1, or DCC-4Fbn were analyzed by immunoblotting. Three independent assays were performed in all of the experiments.