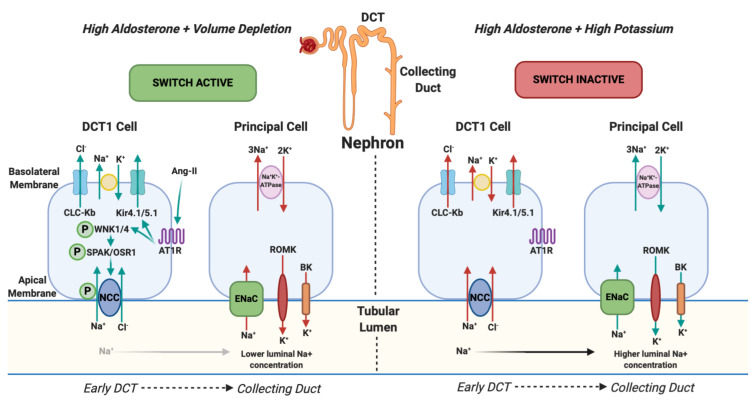
Figure 1. NCC Phosphorylation downstream and changes in urinary potassium (renal potassium switch).
In states of hyperaldosteronism and volume depletion the renal potassium switch is ‘active’. Angiotensin-II (ang-II) stimulates Kir4.1/5.1 to hyperpolarise the basolateral membrane potential and lowers intracellular chloride. This action activates WNK1 (With-no-lysine-kinase-1) and WNK4 (With-no-lysine-kinase-4) by phosphorylation which subsequently phosphorylates SPS1-related proline / alanine-rich kinase (SPAK) and oxidative stress-responsive kinase (OSR1) which, again, phosphorylates the sodium chloride co-symporter (NCC). NCC then avidly reabsorbs sodium (and chloride) back into the body in the early part of the distal convoluted tubule (DCT1). This results in reduced tubular sodium delivery to the collecting duct. Subsequently there is less sodium for the Epithelial sodium Channel (ENaC) to reabsorb and its action is offset. ENaC usually reabsorbs sodium and, via an electrochemical gradient made by sodium-potassium-ATPase, potassium is normally excreted into the tubular lumen by ROMK (Renal Outer Medulla Potassium channel) and BK (Big Potassium/Maxi-K channel) and eliminated from the body. When ENaC is less active, as a consequence of less tubular sodium delivery, less potassium is excreted. This results in a state of hyperaldosteronism with a normal serum potassium. In states of hyperaldosteronism and normal cardiovascular volume (in response to high serum potassium) the switch is ‘inactive’. NCC is not phosphorylated which results in more distal sodium delivery to the collecting duct and subsequent ENaC activation resulting in excretion of potassium into the tubular lumen and a state of hypokalaemia 26, 27. Arrows represent the direction of movement of electrolytes. Red arrows reflect a reduction in movement and green arrows represent an increase in movement. CLC-Kb, chloride voltage-gated channel Kb; Kir4.1/5.1, inwardly rectifying potassium channel 4.1; AT1R, type 1 angiotensin receptor.