Abstract
At the beginning of life, inexperienced babies and human fetuses, domestic chicks, and monkeys exhibit a preference for faces and face-like configurations (three blobs arranged like an upside-down triangle). Because all of these species have parental care, it is not clear whether the early preference for faces is a mechanism for orienting toward the conspecifics and sustaining parental care, or a more general mechanism to attend to living beings. We contrasted these hypotheses by testing inexperienced hatchlings of five species of tortoises, solitary animals with no parental care. If early face-like preference evolved in the context of parental care, solitary species should not exhibit it. We observed that visually naïve tortoises prefer to approach face-like patterns over alternative configurations. The predisposition to approach face-like stimuli observed in hatchlings of these solitary species suggests the presence of an ancient mechanism, ancestral to the evolution of reptiles and mammals, that sustains the exploratory responses, and potentially learning, in both solitary and social species.
Keywords: precocial species, face-like preference, predispositions, neonate, tortoises
A spontaneous preference to orient toward faces and face-like configurations (three blobs arranged as an upside-down triangle inside an ellipse; Fig. 1 A, i) has been observed at the beginning of life in human neonates and fetuses (1–5), domestic chicks, and monkeys (6, 7). To date, this spontaneous preference has been investigated only in social species that rely on early parental care. Hence, a possibility is that the preference for face-like stimuli is an adaptation of social species for orienting toward the conspecifics (e.g., refs. 2, 6, 8) and sustaining parental care. Alternatively, the preference for face-like stimuli might be a behavioral mechanism used to attend to living beings (6, 9), or just a by-product of the architecture of the visual system (10). Only if these alternative hypotheses are correct would one expect solitary species without parental care to show a preference for face-like stimuli at birth. To clarify whether the preference for face-like stimuli depends on parental care, evidence from taxa with no parental care is needed. Land tortoises are a convenient model system to investigate this issue because they can be tested soon after hatching and are solitary: Tortoises of the Testudo genus have no posthatching parental care (11), indicating that, for at least 30 million years, they have evolved with no parental care (12–14), they do not aggregate or form cohesive social groups (15, 16), and hatchlings tend to ignore or avoid conspecifics (17), showing that, from the beginning of life, they are not gregarious. If the preference for face-like stimuli evolved as a behavioral mechanism to enhance parental care or interactions with conspecifics, tortoise hatchlings should not show this preference.
Fig. 1.
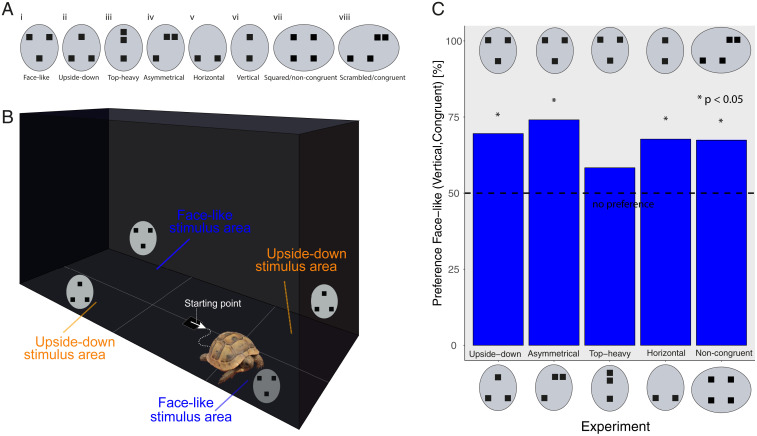
(A) (i) Face-like, (ii) upside-down, (iii) top-heavy, (iv) asymmetrical, (v) horizontal, (vi) vertical, (vii) squared/noncongruent, and (viii) scrambled/congruent to contour. (B) Test apparatus: The subject was located in the starting point facing a short wall, and the first area entered with the entire shell was scored. (C) Preference for the face-like (vertical/congruent) stimulus as percentage of choices.
To test this hypothesis, we measured the first approach responses of naïve tortoise hatchlings of the genus Testudo to face-like stimuli (Fig. 1 A, i) vs. different control stimuli (Fig. 1 A, ii–iv) previously used in the literature (1–6). The first approach is not influenced by experience and is not affected by individual and species differences in speed. To parallel previous studies (2, 4, 8), we used different alternative stimuli: upside-down face-like stimuli to test a preference for the orientation of the configuration, top-heavy stimuli to test the preference for a heavier part of the item in the absence of a triangular configuration, and asymmetrical stimuli to test the preference for the bilateral symmetry of the face-like stimulus. Because the previous experiments indicated a preference for face-like stimuli vs. upside-down and asymmetrical stimuli but not vs. top-heavy stimuli, we ran an experiment to clarify whether vertical patterns elicit a preference over horizontal patterns (Fig. 1 A, v and vi) and an experiment with a squared/noncongruent configuration vs. a scrambled/congruent configuration congruent with the horizontal orientation of the contour (Fig. 1 A, vii and viii).
Results
In the face-like vs. upside-down experiment, we observed a significant preference for the face-like stimuli: 70% (16/23, P = 0.046); in the face-like vs. asymmetrical experiment, we observed a significant preference for the face-like stimulus: 74% (20/27, P = 0.010); in the face-like vs. top-heavy experiment, we observed no significant preference for the face-like stimulus: 56% (14/25, P = 0.345). The last two experiments suggest that the preference might be sustained by top-heavy patterns, with blobs congruent to the contour orientation. In fact, we observed a significant preference for the vertical vs. horizontal stimulus (68%: 21/31, P = 0.035) and for the scrambled/congruent vs. squared/noncongruent stimulus (66%: 20/30, P = 0.049). See results in Fig. 1C.
Discussion
We tested whether the spontaneous attraction for face-like stimuli found in social species with parental care, such as human beings (2, 4, 8), monkeys (7), and domestic chicks (6), is present also in land tortoises, that are solitary animals with no parental care. Surprisingly, naïve tortoise hatchlings exhibited a preference for face-like configurations too. We show that the preference for face-like stimuli is present in solitary species at the beginning of life. These results suggest that the predisposition to orient toward faces/face-like stimuli is not an adaptation for parental care or for sustaining engagement with conspecifics. Then, what is the functional value of this trait, if any? A possibility is that tortoises are attracted to cues associated with living animals, such as face-like stimuli, because living animals provide relevant information, such as the availability of resources. Predispositions might be mechanisms to enhance the acquisition of information from other animals (9). Indirect evidence for this explanation comes from the fact that tortoise hatchlings initially explore unfamiliar individuals before actively moving away from them (17). Another possibility is that this predisposition has no functional adaptive value but derives from a sensitivity of the visual system to top-heavy patterns congruent with the orientation of the bounded area delimiting the features (see ref. 10). Although this explanation is in line with the preference for patterns congruent with the contour, it does not account for the face-like vs. upside-down preference. Briefly, we showed that tortoise hatchlings can discriminate between different configurations of blobs and that the preference for face-like stimuli is not limited to mammals and birds (6) but extends to reptiles. This suggests the presence of an ancient mechanism for orienting toward face-like patterns, evolved in the common ancestors of mammals, reptiles, and birds more than 300 million years ago (18), possibly from a bias toward top-heavy, symmetrical stimuli congruent with the orientation of the outline. Our research calls for further studies to test the hypothesis that predispositions present at the beginning of life are mechanisms that enhance exploration and learning in both solitary and social species.
Materials and Methods
We conducted the experiments in the tortoise sanctuary SperimentArea (Fondazione Museo Civico Rovereto). The experiments were approved by the ethical committee of Fondazione Museo Civico Rovereto and comply with the European Union regulations. We tested 136 tortoise hatchlings in five experiments (Table 1). Although this sample is relatively small, due to the difficulties in spotting egg laying, it is large enough to detect a medium effect size.
Table 1.
Number of face-like (vertical, congruent) choices/overall tortoises in each experiment
| Experiment | Species (Testudo) | ||||
| T. graeca | T. hermanni | T. horsfieldii | T. marginata | Hybrid (T. graeca x T. marginata) | |
| Face-like vs. upside-down | 8/12 | 8/11 | 0 | 0 | 0 |
| Face-like vs. top-heavy | 6/9 | 0 | 2/5 | 6/11 | 0 |
| Face-like vs. asymmetrical | 6/7 | 2/5 | 3/5 | 4/4 | 5/6 |
| Vertical vs. horizontal | 6/7 | 5/7 | 2/6 | 4/5 | 4/6 |
| Congruent vs. squared | 4/8 | 3/6 | 6/6 | 3/4 | 4/6 |
Eggs buried underground by tortoises were collected and incubated in darkness at 31 ± 2 °C for 55 d to 60 d, until hatching. Before hatching, eggs were located in opaque, individual compartments in darkness. Soon after hatching, each subject was housed in a 15 × 15 × 12 cm opaque box, with the bottom covered with soil, leaves, and straw, exposed to the day/night cycle outdoors. Each subject was fed with green leaves and hydrated as needed. During animal care and experiments, we covered our faces with uniform masks to ensure that tortoises did not see any face before the test.
Stimuli were cardboard ellipses (3.1 × 2.5 cm or 2.5 × 4 cm) with light gray background and black square blobs (0.4 × 0.4 cm) (Fig. 1A). The face-like stimulus contained three blobs arranged in an upside-down isosceles triangle placed in the center of the ellipse. The upside-down stimulus presented the face-like pattern rotated by 180°. The top-heavy stimulus contained three central blobs vertically located, with the average height of the top blobs equal to the face-like stimulus. The asymmetrical stimulus contained three blobs arranged in a scrambled triangle. The horizontal stimulus contained two bottom blobs, and the vertical stimulus contained two vertical blobs. The right/left position of the stimuli was counterbalanced between subjects.
We tested tortoises in a rectangular arena (30 × 20 × 28 cm) with four stimuli located on the edges of the long sides in four areas (Fig. 1B). At test, the experimenter gently placed the subject in the starting position in the middle of the arena, facing a short wall, with two different stimuli visible on the opposite sides (Movie S1). A camera recorded the test from above. Before each trial, the apparatus was cleaned and placed under a halogen lamp (400 W). Before the test, experimenters thermoregulated the subject by exposing its box to the sun (under a lamp in case of overcast sky), until the animal walked spontaneously. Subjects’ left/right position was counterbalanced. If the tortoise did not enter more than one area after 25 min the session was repeated after at least 24 h. We scored the first area entered with the entire shell and analyzed the preference at the population level using a binomial test. Significance was set at P < 0.05. The interrater agreement between the experimenter and a second person blind to the experimental conditions and hypotheses was 100% (calculated on 65 trials of all experiments).
Supplementary Material
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011453117/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Goren C. C., Sarty M., Wu P. Y., Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics 56, 544–549 (1975). [PubMed] [Google Scholar]
- 2.Morton J., Johnson M. H., CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychol. Rev. 98, 164–181 (1991). [DOI] [PubMed] [Google Scholar]
- 3.Reid V. M. et al., The human fetus preferentially engages with face-like visual stimuli. Curr. Biol. 27, 1825–1828.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Simion F., Di Giorgio E., Face perception and processing in early infancy: Inborn predispositions and developmental changes. Front. Psychol. 6, 969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buiatti M. et al., Cortical route for facelike pattern processing in human newborns. Proc. Natl. Acad. Sci. U.S.A. 116, 4625–4630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giorgio E. et al., Filial responses as predisposed and learned preferences: Early attachment in chicks and babies. Behav. Brain Res. 325, 90–104 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Sugita Y., Face perception in monkeys reared with no exposure to faces. Proc. Natl. Acad. Sci. U.S.A. 105, 394–398 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson M. H., Senju A., Tomalski P., The two-process theory of face processing: Modifications based on two decades of data from infants and adults. Neurosci. Biobehav. Rev. 50, 169–179 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Versace E., Martinho-Truswell A., Kacelnik A., Vallortigara G., Priors in animal and artificial intelligence: Where does learning begin? Trends Cogn. Sci. 22, 963–965 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Simion F., Macchi Cassia V., Turati C., Valenza E., The origins of face perception: Specific versus non-specific mechanisms. Infant Child Dev. 10, 59–65 (2001). [Google Scholar]
- 11.Diaz-Paniagua C., Keller C., Andreu A. C., Clutch frequency, egg and clutch characteristics, and nesting activity of spur-thighed tortoises, Testudo graeca, in southwestern Spain. Can. J. Zool. 74, 560–564 (1996). [Google Scholar]
- 12.Parham J. F. et al., The phylogeny of Mediterranean tortoises and their close relatives based on complete mitochondrial genome sequences from museum specimens. Mol. Phylogenet. Evol. 38, 50–64 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Joyce W. G., Parham J. F., Lyson T. R., Warnock R. C. M., Donoghue P. C. J., A divergence dating analysis of turtles using fossil calibrations: An example of best practices. J. Paleontol. 87, 612–634 (2013). [Google Scholar]
- 14.de Lapparent de Broin F., Bour R., Parham J. F., Perälä J., Eurotestudo, a new genus for the species Testudo hermanni Gmelin, 1789 (Chelonii, Testudinidae). Syst. Paleontol. Vertebrate Paleontol. 5, 803–811 (2006). [Google Scholar]
- 15.Ernst C. H., Barbour R. W., Turtles of the World, (Smithsonian Institution Press, 1989). [Google Scholar]
- 16.Pearse D. E., Avise J. C., Turtle mating systems: Behavior, sperm storage, and genetic paternity. J. Hered. 92, 206–211 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Versace E., Damini S., Caffini M., Stancher G., Born to be asocial: Newly-hatched tortoises spontaneously avoid unfamiliar individuals. Anim. Behav. 138, 152314 (2018). [Google Scholar]
- 18.Naumann R. K. et al., The reptilian brain. Curr. Biol. 25, R317–R321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.