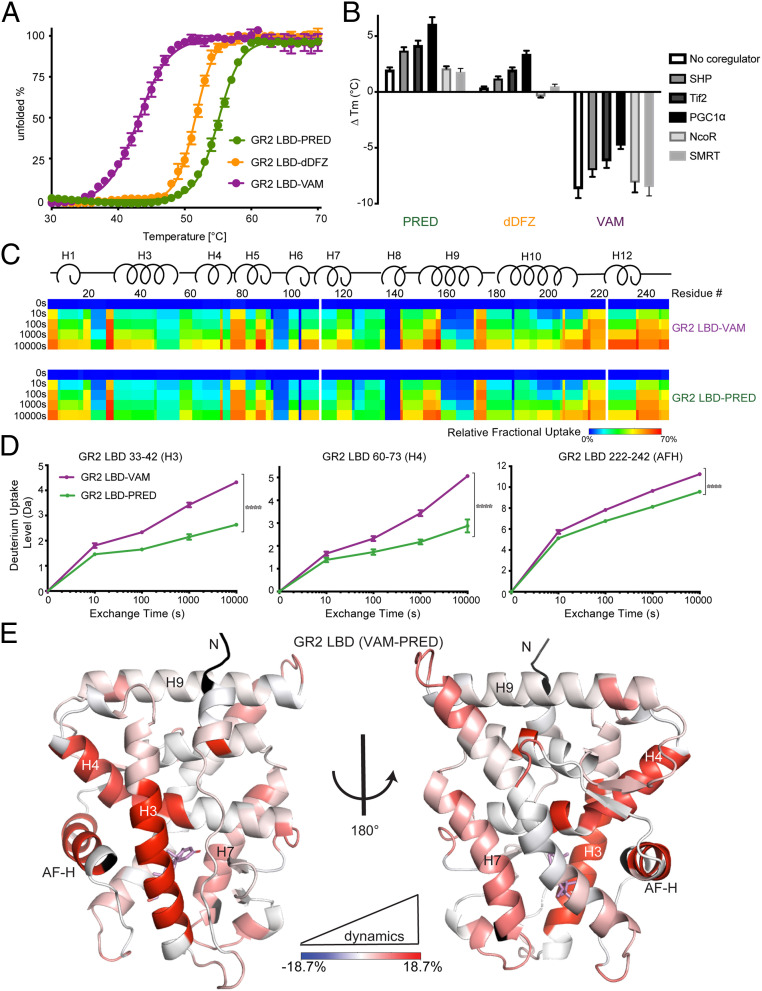
Fig. 4.
AncGR2 LBD with VAM has reduced stability and enhanced protein dynamics. (A) Thermal unfolding curves of AncGR2 LBD bound to PRED, dDFZ, and VAM. (B) Difference in thermostability (∆Tm) of GR2 LBD with different ligands in the presence of coregulators compared to GR2 LBD with HCY as expressed by ∆Tm = [Tm(ligand) − Tm(HCY)] for each coregulator. (C). Heat maps of deuterium uptake monitored by HDX-MS for AncGR2 LBD bound to PRED and VAM. Different time points of LBD incubation in D2O before measuring deuterium uptake are indicated on the Left. (D) Three representative HDX plots of peptic fragments from PRED- and VAM-bound AncGR2 LBD. (E) Differential deuterium uptakes are mapped on the structure of AncGR2 LBD in complex with VAM. Residues are colored in a continuous gradient from blue to red, with their intensity scaling to the difference in percentage of deuterium exchange [(VAM-bound) − (PRED-bound)]. Residues not covered by any peptides are shown in black.