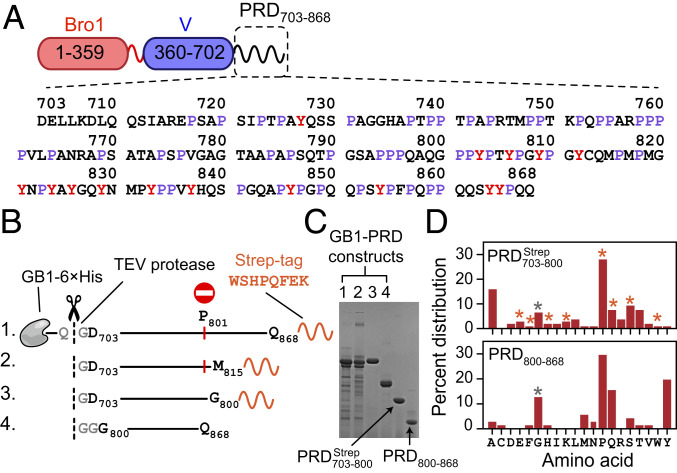
Fig. 1.
ALIX domain organization and summary of ALIX-PRD constructs used in the current work. (A) Schematic of ALIX organization. Primary sequence of PRD is shown with prolines (∼30%) and tyrosines (∼9%) labeled in purple and red, respectively. (B) Recombinant PRD constructs, namely GB1– GB1– GB1– and GB1–PRD800–868. The positions of purification tags, 6×His and strep, are marked (primary sequence of strep tag is shown). TEV protease cutting sites are shown in gray and marked with dashed lines and scissors. Recombinant expression of GB1– and GB1– resulted in truncated fragments because of ribosomal stalling induced by polyproline stretches, especially at residue P801, marked by a red circle and vertical red line. (C) SDS-PAGE analysis of purified PRD constructs [16% wt/vol tris(hydroxymethyl)aminomethane–glycine gel]; the order of GB1–PRD fusion constructs is the same as the one depicted in B. TEV-cleaved products, namely and PRD800–868, are marked with arrows. (D) Amino acid composition of (Top) and PRD800–868 (Bottom). Vertical bars marked with orange and gray asterisks denote contributions from nonnative strep tag and remnant-glycine residues of TEV cleavage sites, respectively.