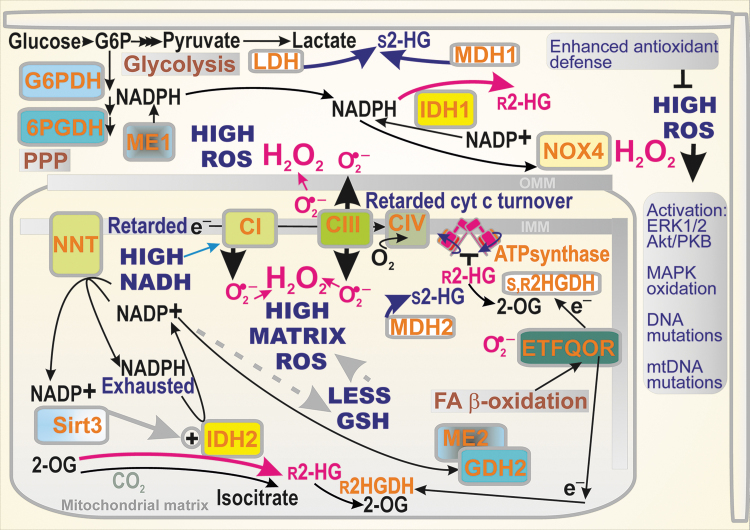
FIG. 3.
Consequences of IDH1/2-related cancer metabolism for redox homeostasis. A simplified scheme demonstrates the pro-oxidant character of reductive reactions of IDH1 and IDH2. Retarded electron (e−) transfer via the respiratory chain complexes CI, CIII, and retarded turnover of cytochrome c, together with feedback inhibition of proton pumping by the inhibited ATP-synthase with r-2HG (hypothetically also in humans) are the main inducers of elevated superoxide (O2•−) formation. In particular, a high NADH/NAD+ ratio leads to superoxide formation at the flavin IF site of Complex I (not shown). Superoxide is dismuted by the matrix MnSOD/SOD2 and intermembrane space or cytosolic CuZnSOD/SOD1 (data not shown) into H2O2, the most prominent ROS. High ROS formation within the mitochondrial matrix depletes GSH, which requires NADPH for its synthesis. As a result, NADPH is instead depleted or rapidly diminished. The oxidated mitochondrial ROS equilibrium is spread toward the cytosol. Also, fast tumor growth contributes to depletion of cytosolic NADPH. Its pool is regenerated when cytosolic NAPDH is supplied by two PPP enzymes, G6PDH and 6PGDH but consumed again by the constitutively active NADPH oxidase NOX4 that produces H2O2 directly. Both r-2HG and s-2HG can stimulate not only the NRF2-reprogrammed elevated antioxidant defense that attenuates the cytosolic but also mitochondrial oxidative stress. Nevertheless, ROS being elevated even transiently can evoke the activation of redox-sensitive kinases, besides oxidizing DNA and mtDNA. 6PGDH, 6-phosphogluconate dehydrogenase; G6PDH, glucose-6-phosphate dehydrogenase; GSH, reduced glutathione; H2O2, hydrogen peroxide; mtDNA, mitochondrial DNA; NOX4, NADPH oxidase isoform 4; NRF2, nuclear factor erythroid 2-related factor; PPP, pentose phosphate pathway; ROS, reactive oxygen species; SOD, superoxide dismutase.