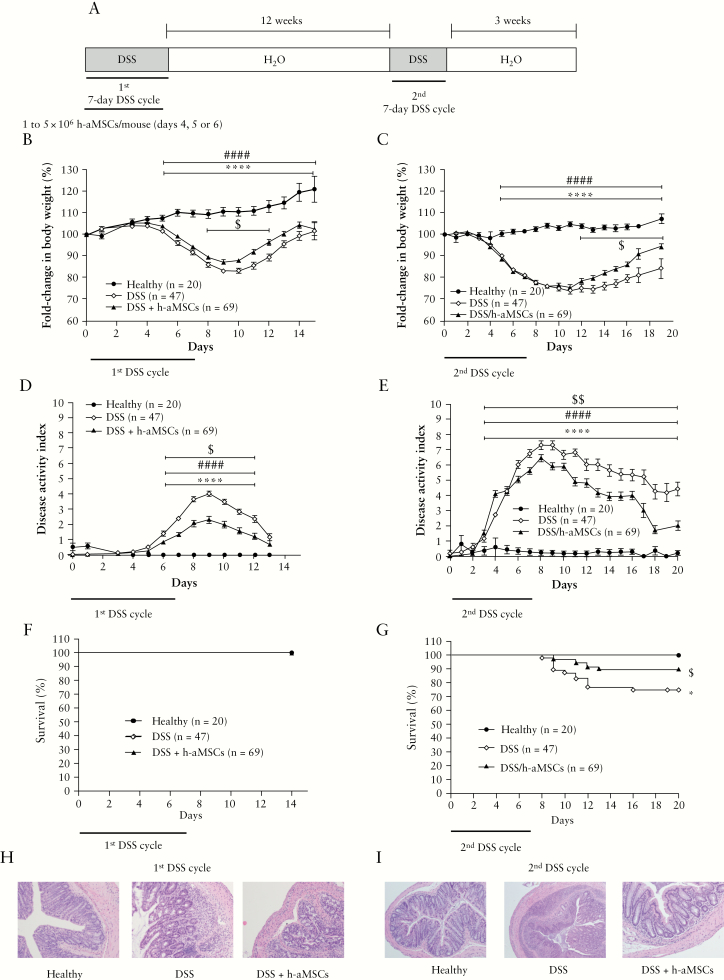
Figure 1.
Experimental design and DSS-induced colitis status of mice following intraperitoneal administration of human adipose-derived MSCs. Experimental design of DSS-induced colitis; [A] fold-change in body weight during the first [B] and the second [C] treatment with DSS; disease activity index during the first [D] and the second [E] treatment with DSS; survival during the first [F] and the second [G] treatment with DSS. Representative images of colon tissue [magnification 100X] at Day 9 following the first [H] and at Day 10 upon the second [I] treatment with DSS. Healthy, n = 20; DSS, n = 47, and DSS + h-aMSCs, n = 69. Data are presented by mean and standard error of the disease activity index and of the fold-change in body weights, with respect to Day 0 expressed as percentage of initial weight. Survival data are presented by percentage. Significance was analysed by the Mann-Whitney U test and log rank test and represented by *p <0.05 and ****p ≤0.0001 DSS vs heathy, ####p ≤0.0001 DSS + h-aMSCs vs healthy, and $p <0.05 and $$p <0.01 DSS + h-aMSCs vs DSS. Results correspond to 12 independent experiments. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.