Figure 6. Zwitterionic micelle/insulin aqueous formulation loses pharmacological activity through direct oral administration but shows stability to form dry powders for potentially oral capsule formulation.
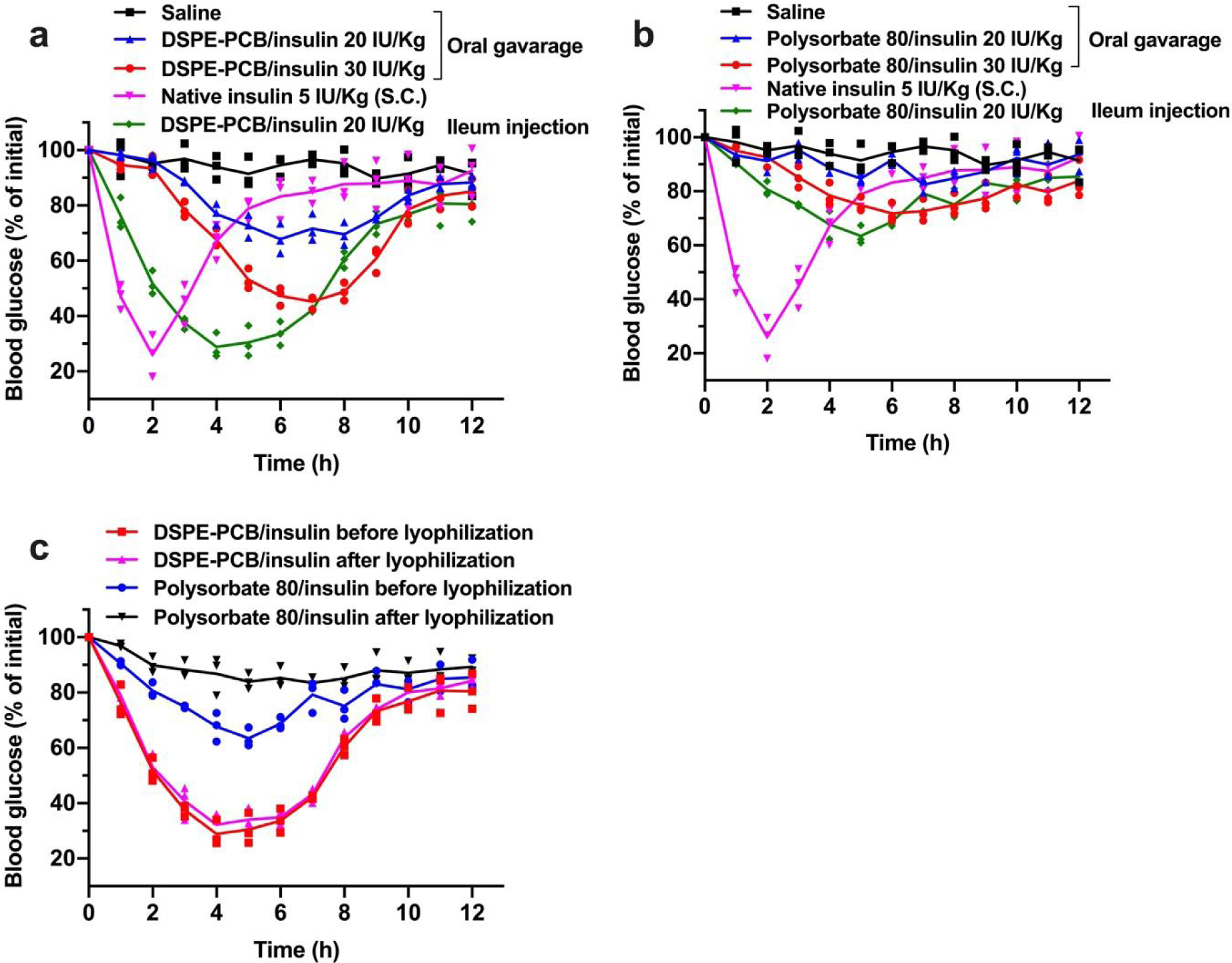
Blood glucose lowering (pharmacological) performance for (a) DSPE-PCB/insulin and (b) polysorbate 80/insulin aqueous formulation on diabetic mice through oral gavage at 20 and 30 IU/Kg compared with ileum injection at 20 IU/kg (N=3 biologically independent animals, means connected). Insulin/ZnCl2 feeding ratio is 2.5/1 by weight. S.c. injected native insulin at 5 IU/Kg was used as a control (1 IU/ml). (c) Blood glucose lowering (pharmacological) performance for DSPE-PCB/insulin and polysorbate 80/insulin aqueous formulation on diabetic mice through ileum injection at 20 IU/Kg before and after a lyophilization procedure (N=3 biologically independent animals, means connected).
