Abstract
Background
Trimethylamine-N-Oxide (TMAO) is a proatherogenic and prothrombotic metabolite. Our study examined the association of plasma TMAO level with cardiovascular and all-cause mortality in hemodialysis (HD) patients.
Methods
Patients who were at least 18 years-old and received HD for at least 6 months were enrolled within 6 months. Patients with coronary heart disease, congestive heart failure, arrhythmia, or stroke within 3 months before study onset were excluded. The primary endpoints were cardiovascular and all-cause death, and the secondary endpoint was cerebrovascular death.
Results
We recruited 252 patients and divided them into a high-TMAO group (>4.73 μg/mL) and a low-TMAO group (≤4.73 μg/mL). The median follow-up time was 73.4 months (interquartile range: 42.9, 108). A total of 123 patients died, 39 from cardiovascular disease, 19 from cerebrovascular disease, and 65 from other causes. Kaplan-Meier analysis indicated that the high-TMAO group had a greater incidence of cardiovascular death (Log-Rank: p = 0.006) and all-cause death (Log-Rank: p < 0.001). Cox regression analysis showed that high TMAO level was significantly associated with cardiovascular and all-cause mortality. After adjustment for confounding, this association remained significant for cardiovascular mortality (TMAO as a continuous variable: HR: 1.18, 95%CI: 1.07, 1.294, p < 0.001; TMAO as a dichotomous variable: HR: 3.44, 95%CI: 1.68, 7.08, p < 0.001) and all-cause mortality (TMAO as a continuous variable: HR: 1.14, 95%CI: 1.08, 1.21, p < 0.001; TMAO as a dichotomous variable: HR: 2.54, 95%CI: 1.71, 3.76, p < 0.001).
Conclusions
High plasma TMAO level is significantly and independently associated with cardiovascular and all-cause mortality in HD patients.
Keywords: Uremic toxins, trimethylamine N-oxide, cardiovascular mortality, all-cause mortality, hemodialysis
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in patients receiving hemodialysis (HD) [1]. However, the specific factors that lead to CVD in these patients are unknown [2]. Traditional risk factors for CVD in the general population, such as diabetes, hypertension, left ventricular hypertrophy, hypertriglyceridemia, do not fully explain the increased risk of CVD in HD patients [3,4].
Uremic toxins, such as indoxyl sulfate and p‐cresyl sulfate, increase the risk of CVD in HD patients [5–7]. Recent pioneering research identified the metabolite trimethylamine-N-Oxide (TMAO) as strongly proatherosclerotic and prothrombotic [8–10]. Extensive epidemiological studies of non-dialysis patients found that plasma TMAO level was associated with CVD, and that TMAO was a predictor of major adverse cardiovascular events. In particular, after adjusting for traditional CVD risk factors and renal function, a higher plasma TMAO concentration was associated with a 2.5-fold increased risk of cardiovascular events [9]. Multiple research groups worldwide have confirmed the association of TMAO with CVD, and are gradually elucidating the possible mechanism by which TMAO leads to atherosclerosis and thrombosis [8,11–14].
There is also increasing evidence that elevated plasma TMAO level is associated with poor cardiovascular outcomes in patients with chronic kidney disease (CKD) [15–17]. The plasma TMAO levels in HD patients are more than 20-times higher than in individuals with normal renal function [15,18]. However, the relationship between TMAO and cardiovascular outcomes in HD patients is uncertain. Some studies found that the association of TMAO with poor cardiovascular outcomes in HD patients varied among different ethnic groups, but other studies failed to find such relationships [19–21].
In 2009, we established a prospective cohort to study plasma uremic toxins and cardiovascular outcomes in HD patients. We measured plasma TMAO in blood samples from this cohort during 2018. The goal of the present study was to analyze the longitudinal association of TMAO with cardiovascular and all-cause mortality in HD patients. All enrolled patients had minimal or no residual kidney function, thus providing an opportunity to determine if this association exists without potential confounding from residual renal function.
Materials and methods
Study population
Patients (≥18 years old) who were on maintenance HD for at least 6 months were recruited from the Blood Purification Center of Zhongshan Hospital, Fudan University. Patients with angina pectoris, acute myocardial infarction, arrhythmia, congestive heart failure, or stroke within 3 months before study onset were excluded. Compared to our 2015 study of the same cohort [5], 6 patients who experienced episodes of arrhythmias 3 months before study onset were excluded, including 5 with atrial fibrillation and 1 with a premature ventricular beat. Patients were enrolled from July to December of 2009. The study adhered to the Declaration of Helsinki and was approved by the Ethical Committee of Zhongshan Hospital, Fudan University (NO. 2008-114). All participants provided written informed consent.
All participants received HD three times per week using the prescription described previously [5]. Dry weight targeting of each participant was used to achieve an edema-free state and was reevaluated monthly. All patients were advised to consume high-protein diets (at least 1.2 g/kg per day of mainly animal protein).
Anthropometric measurements, blood sampling, clinical data collection, and biochemical measurements
The protocol for the collection of blood samples was described previously [5]. Blood samples were collected on a midweek nondialysis day (Thursday for those receiving dialysis on Monday/Wednesday/Friday; Friday for those receiving dialysis on Tuesday/Thursday/Saturday) from 8:00 to 10:00 a.m to avoid the influence of dialysis and blood volume on the TMAO concentration. Demographic and clinical data were collected, including gender, age, height, weight, dialysis duration, smoking history, underlying kidney disease, comorbidities, and use of medications. Blood pressure, average interdialytic weight gain, and ultrafiltration volume were obtained by averaging all predialysis blood pressure, interdialytic weight gain, and ultrafiltration volume values during the 4 weeks (12 times in total) before the study. Urinary volume was measured, and residual renal function was determined from the estimated glomerular filtration rate (eGFR) as a mean of urea and creatinine clearance (mL/min/1.73 m2). The normalized protein nitrogen appearance rate (nPNA) was calculated as described by Depner and Daugirdas [22]. Biochemical evaluations were measured using standard methods in the clinical laboratory.
TMAO quantification
We measured TMAO in plasma by liquid chromatography/mass spectrometry/mass spectrometry using TMAO-D9 (Toronto Research Chemicals, Canada) as the internal standard [21]. 100 μL plasma was deproteinized by mixture with 500 μL methanol containing TMAO-D9 (internal standard solution) at the concentration of 1 µg/mL. Then, the samples were centrifuged at 12,000 × g for 10 min. After that, 100 μL supernatants were transferred, diluted with an equal volume of distilled water and placed in autosampler at 4 °C. Then 10 μL of each sample supernatant was injected in Agilent 1100 HPLC system (Agilent, USA), and analytes were separated on a column (100 mm × 2.1 mm, 5 µm, Venusil XBP Phenyl column; Phenomenex, Torrance, CA) at 40 °C. The mobile phase was 0.1% formic acid (v/v) and the methanol flow rate was 0.4 mL/min. Mass spectrometry was performed on an API 3000 Triple Quadrupole Mass Spectrometer (Applied Biosystems, USA) with electrospray ionization in the positive mode, and ion transitions used for quantitation were m/z 76.0→59.0 for TMAO and m/z 85.1→68.0 for TMAO-D9.
End point evaluation
The primary endpoints were cardiovascular and all-cause death. The secondary endpoint was cerebrovascular death. The diagnostic criteria for cardiovascular death were the direct cause of death from myocardial infarction, heart failure, arrhythmia, or sudden cardiac death. Patients were followed up until 31 December 2018. A group of clinicians who were blinded to the TMAO data were responsible for diagnosing and recording clinical events, including heart failure, angina pectoris, acute myocardial infarction, arrhythmia, stroke, cardiac death, and noncardiac death. All patients received weekly follow-ups for the evaluation of clinical events. If a patient was hospitalized, visited the emergency department, or needed emergency HD, the doctor on duty recorded it on the patient’s chart and submitted it to the department for case discussion on Friday afternoon.
Statistical analyses
Data are expressed as means ± SDs for variables with normal distributions, medians (interquartile ranges) for variables with skewed distributions, and frequencies for categorical variables. Statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL). Differences were considered significant when a two-tailed p-value was below 0.05. Baseline variables were compared using a chi-squared test for categorical variables, a t-test for continuous variables that had Gaussian distributions, and a Mann-Whitney test for continuous variables that had non-Gaussian distributions. Kaplan-Meier method and Cox proportional hazard model were used to estimate the association of TMAO with cardiovascular and all-cause mortality. The Log-Rank test was used to compare different Kaplan-Meier curves. Univariate and multivariate analyses of cardiovascular and all-cause mortality were performed using a Cox proportional hazard model as a function of plasma TMAO when it was treated as a continuous variable or a dichotomous variable.
Eight models were used to assess the risk for cardiovascular and all-cause mortality with adjustment for confounding by different factors. In model 8, hierarchical selection procedures were used, in which the inclusion criteria for model selection in the covariate set was predetermined as a p-value below 0.10 in the univariate Cox proportional hazard model. TMAO was entered into each model as a continuous or dichotomous variable.
Results
The study cohort consisted of 252 HD patients (141 men), the mean age was 57.1 ± 14.5 years, and glomerular disease was the leading cause of end-stage renal disease (44.4%). Among all patients, 31.3% had primary hypertension, 6.7% had coronary heart disease, and 12.3% had diabetes. There was an extraordinarily wide range of TMAO concentrations (0.41–18.7 μg/mL). Based on the median value, we categorized patients as having a low TMAO (≤4.73 μg/mL) or a high TMAO (>4.73 μg/mL). The high-TMAO group was older, had a higher concentration of β2-microglobulin, and was less likely to use calcitriol. Among the four leading causes of the underlying kidney disease (glomerular disease, hypertension, diabetes, polycystic kidney disease), only diabetes had a higher prevalence in the high-TMAO group. The two groups were similar in other characteristics (Table 1).
Table 1.
Baseline demographic, clinical, and biochemical characteristics of 252 HD patients.
| All patients | Low TMAO group (≤4.73 μg/ml) | High TMAO group (>4.73 μg/ml) | p Value | |
|---|---|---|---|---|
| n = 252 | n = 126 | n = 126 | ||
| Demographics | ||||
| Age (years) | 57.1 ± 14.5 | 55.1 ± 15.6 | 59.1 ± 13.1 | 0.03 |
| Male sex | 141 (56.0) | 75 (59.5) | 66 (52.4) | 0.25 |
| BMI (kg/m2) | 22.2 ± 3.4 | 21.9 ± 3.6 | 22.5 ± 3.1 | 0.14 |
| Dialysis characteristics | ||||
| Duration (months) | 43.0 (19.3–74.5) | 37.5 (15.0–76.5) | 46.0 (26.8–70.5) | 0.17 |
| Interdialytic weight gain (%) | 3.2 ± 1.2 | 3.2 ± 1.1 | 3.2 ± 1.0 | 0.76 |
| Ultrafiltration volume (mL) | 2001 ± 748 | 1994 ± 782 | 2032 ± 704 | 0.68 |
| Systolic blood pressure (mmHg) | 136.7 ± 17.7 | 135.1 ± 17.0 | 138.3 ± 18.4 | 0.15 |
| Diastolic blood pressure (mmHg) | 82.3 ± 10.5 | 82.3 ± 10.4 | 82.3 ± 10.6 | 0.97 |
| Single-pool Kt/V | 1.40 ± 0.22 | 1.40 ± 0.21 | 1.41 ± 0.02 | 0.93 |
| Urinary volume (mL/kg per 24 h) | 0 (0–5.2) | 0.3 (0–7.4) | 0.2 (0.5.2) | 0.48 |
| eGFR (mL/min/1.73 m2) | 0 (0–0.5) | 0 (0–0.6) | 0 (0–0.4) | 0.34 |
| Normalized protein nitrogen appearance (g/kg per day) | 2.2 ± 0.6 | 2.1 ± 0.4 | 2.2 ± 0.5 | 0.16 |
| Smoking history (n, %) | 93 (36.9%) | 47 (37.3%) | 46 (36.5%) | 0.90 |
| Underlying kidney disease | 0.04 | |||
| Glomerular disease (n, %) | 112 (44.4%) | 56 (44.4%) | 56 (44.4%) | |
| Diabetic nephropathy (n, %) | 21 (8.3%) | 5 (4.0%) | 16 (12.7) | |
| Hypertensive nephropathy (n, %) | 20 (7.9%) | 12 (9.5%) | 8 (6.3%) | |
| Polycystic kidney disease (n, %) | 18 (7.1%) | 12 (9.5%) | 6 (4.8) | |
| Others (n, %) | 38 (15.1%) | 23 (18.3%) | 15 (11.9%) | |
| Unknown (n, %) | 43 (17.1%) | 18 (14.3%) | 25 (19.8%) | |
| Comorbidity | ||||
| Primary hypertension (n, %) | 79 (31.3%) | 40 (31.7%) | 39 (31.0%) | 0.89 |
| Coronary heart disease (n, %) | 17 (6.7%) | 9 (7.1%) | 8 (6.3%) | 0.80 |
| Diabetes (n, %) | 31 (12.3%) | 9 (7.1%) | 22 (17.5%) | 0.01 |
| Cerebral infarction (n, %) | 36 (14.3%) | 14 (11.1%) | 22 (17.5%) | 0.15 |
| Cerebral hemorrhage (n, %) | 8 (3.2%) | 5 (4.0%) | 3 (2.4%) | 0.47 |
| Gout (n, %) | 55 (21.8%) | 30 (23.8%) | 25 (19.8%) | 0.45 |
| Medications (%) | ||||
| CCB (n, %) | 156 (61.9%) | 75 (59.5%) | 81 (64.3%) | 0.44 |
| ACEI (n, %) | 42 (16.7%) | 19 (15.1%) | 23 (18.3%) | 0.50 |
| ARB (n, %) | 67 (26.6%) | 36 (28.6%) | 31 (24.6%) | 0.48 |
| β-Blocker (n, %) | 40 (15.9%) | 22 (17.5%) | 18 (14.3%) | 0.49 |
| α-Blocker (n, %) | 46 (18.3%) | 21 (16.7%) | 25 (19.8) | 0.51 |
| Aspirin (n, %) | 51 (20.2%) | 22 (17.5%) | 29 (23.0%) | 0.27 |
| Statin (n, %) | 14 (5.6%) | 6 (4.8%) | 8 (6.3%) | 0.58 |
| Calcium supplement (n, %) | 166 (65.9%) | 83 (65.9%) | 83 (65.9%) | 1.0 |
| Calcitriol (n, %) | 137 (54.4%) | 77 (61.1%) | 60 (47.6%) | 0.03 |
| Predialysis laboratory tests | ||||
| Albumin (g/L) | 39.2 ± 3.7 | 39.1 ± 3.8 | 39.3 ± 3.6 | 0.55 |
| Prealbumin (g/L) | 0.33 ± 0.09 | 0.33 ± 0.08 | 0.33 ± 0.09 | 0.53 |
| Hemoglobin (g/L) | 103.1 ± 16.0 | 103.9 ± 17.6 | 102.3 ± 14.3 | 0.42 |
| BUN (mmol/L) | 23.9 ± 5.4 | 23.5 ± 5.1 | 24.3 ± 5.7 | 0.25 |
| Serum creatinine (μmol/L) | 1020.7 ± 261.2 | 1012.7 ± 261.2 | 1028.8 ± 262.0 | 0.63 |
| Uric acid (μmol/L) | 434.9 ± 85.3 | 431.6 ± 78.2 | 438.2 ± 92.0 | 0.54 |
| Calcium (mmol/L) | 2.22 ± 0.21 | 2.22 ± 0.18 | 2.21 ± 0.23 | 0.58 |
| Phosphorus (mmol/L) | 2.17 ± 0.63 | 2.16 ± 0.66 | 2.18 ± 0.61 | 0.86 |
| Alkaline phosphatase (U/L) | 72.0 (56.0–92.5) | 72.0 (55.0–100.0) | 73.0 (56.0–87.5) | 0.78 |
| 25-Hydroxy vitamin D (nmol/L) | 57.5 ± 19.0 | 56.3 ± 18.9 | 58.8 ± 19.0 | 0.31 |
| iPTH (pg/mL) | 272.9 (137.0–553.6) | 271.5 (139.5–552.8) | 278.5 (136.7–565.9) | 0.84 |
| UIBC (μmol/L) | 25 (20–29) | 24 (21–28) | 26 (20–30) | 0.40 |
| TIBC (μmol/L) | 36 (32–41) | 36 (31–41) | 36 (33–40) | 0.52 |
| Iron (μmol/L) | 10.5 (7.1–14.2) | 10.6 (7.7–14.9) | 10.0 (7.0–13.8) | 0.60 |
| Transferrin (g/L) | 1.9 (1.6–2.1) | 1.8 (1.6–2.1) | 1.9 (1.7–2.2) | 0.23 |
| Ferritin (ng/mL) | 126.8 (68.5–265.2) | 124.6 (70.1–262.1) | 128.6 (61.6–282.3) | 0.72 |
| hsCRP (mg/L) | 2.1 (0.7–6.3) | 2.1 (0.7–6.2) | 2.2 (0.8–7.0) | 0.37 |
| Triglycerides (mmol/L) | 1.4 (1.1–1.9) | 1.4 (1.1–1.9) | 1.4 (1.1–1.9) | 0.55 |
| Total cholesterol (mmol/L) | 4.4 ± 1.1 | 4.3 ± 1.1 | 4.4 ± 1.2 | 0.31 |
| HDL-C (mmol/L) | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.37 |
| LDL-C (mmol/L) | 2.5 ± 0.9 | 2.4 ± 0.8 | 2.5 ± 0.9 | 0.38 |
| Apo-A (g/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.84 |
| Apo-B (g/L) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.40 |
| Lp (a) (mg/L) | 173 (115–281) | 178 (115–309) | 169 (115–251) | 0.21 |
| Homocysteine (μmol/L) | 34.7 (27.8–43.1) | 34.6 (27.9–43.1) | 35.1 (27.7–43.1) | 0.98 |
| β2-Microglobulin (mg/L) | 35.8 ± 11.4 | 34.0 ± 11.6 | 37.7 ± 11.0 | 0.008 |
| TMAO (μg//ml) | 4.73 (3.20–6.69) | 3.30 (2.25–4.00) | 6.67 (5.54–8.65) | <0.001 |
Data are presented as means ± SDs or medians (interquartile ranges) for continuous variables and as n (%) for categorical variables.
Abbreviations here and below: ACEI: angiotensin-converting-enzyme inhibitor; Apo: apolipoprotein; ARB: angiotensin II receptor blocker; BMI: body mass index; BUN: blood urea nitrogen; CCB: calcium channel blocker; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; hsCRP: high-sensitivity C-reactive protein; iPTH: intact parathyroid hormone; LDL-C: low-density lipoprotein cholesterol; Lp: lipoprotein; TIBC: total-iron binding capacity; UIBC: unsaturated iron-binding capacity.
The bold values represents as Age, underlying kidney disease, Comorbid of diabetes, calcitriol (active vitamin D), β2-Microglobulin, are related to the patients' outcomes, and we developed eight different multivariate models to adjust for these confounders. TMAO is the factor we aimed to examine whether it is associated with cardiovascular death and all-cause death.
The median follow-up time was 73.4 months (interquartile range [IQR]: 42.9–108). During follow-up, 123 patients died, 39 from cardiac death, 19 from cerebrovascular death, and 65 from other causes (sudden death, n = 14; pulmonary infection, n = 14; tumor, n = 11; gastrointestinal bleeding, n = 8; septicemia, n = 4; malnutrition/cachexia, n = 6; multiple organ failure, n = 5; complications secondary to kidney transplantation, n = 1; traumatic brain injury, n = 1; and suicide, n = 1). Twenty patients were transferred to other dialysis centers, and 15 patients received kidney transplantation.
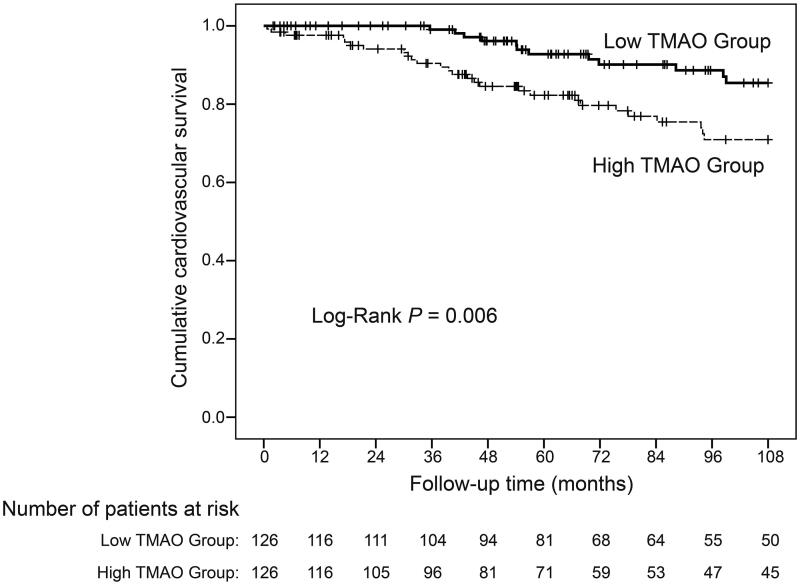
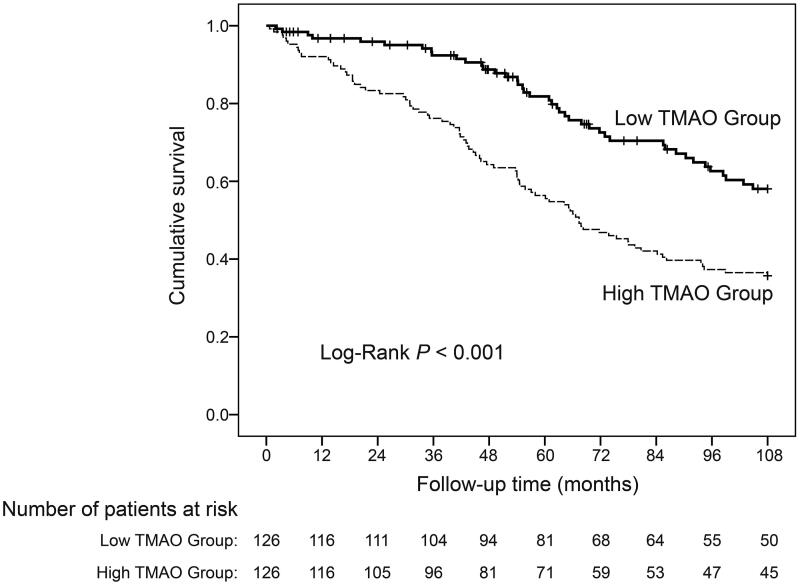
Our initial Kaplan-Meier analyses indicated that the incidences of cardiovascular death (Log-Rank: p = 0.006, Figure 1) and all-cause death (Log-Rank: p < 0.001, Figure 2) were significantly greater in the high-TMAO group. Given that many other risk factors are related to cardiovascular and all-cause death, we constructed Cox proportional hazard models to adjust for these confounding factors. The initial univariate Cox proportional hazard model (Table 2) showed that plasma TMAO was significantly associated with cardiovascular mortality (continuous variable: hazard ratio [HR]: 1.13, 95% confidence interval [95% CI]: 1.03, 1.24, p = 0.01; dichotomous variable: HR: 2.52, 95% CI: 1.28, 4.97, p = 0.008) and all-cause mortality (continuous variable: HR: 1.12, 95% CI: 1.07, 1.18, p < 0.001; dichotomous variable: HR: 2.15, 95% CI: 1.48, 3.12, p < 0.001). As expected, many other variables were also associated with cardiovascular death (age, history of coronary heart disease, history of diabetes, history of cerebral infarction, medication history of calcium supplement and active vitamin D, albumin, prealbumin, iron, and high-sensitivity C-reactive protein [hsCRP]) and with all-cause death (age, history of primary hypertension, history of coronary heart disease, history of diabetes, history of cerebral infarction, medication history of calcium supplement and active vitamin D, albumin, prealbumin, hemoglobin, creatinine, alkaline phosphatase, iron, and hsCRP).
Figure 1.
Kaplan-Meier survival curves of cardiovascular death during follow-up of HD patients stratified by the TMAO level.
Figure 2.
Kaplan-Meier survival curves of all-cause death during follow-up of HD patients stratified by the TMAO level.
Table 2.
Univariate Cox proportional hazard model of variables associated with cardiovascular death and all-cause death in HD patients*.
| Variable | Cardiovascular death |
All-cause death |
|||
|---|---|---|---|---|---|
| Unit of Increase | HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age | 1 year | 1.07 (1.04–1.10) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| Gender | Female vs. male | 1.08 (0.57–2.03) | 0.81 | 1.37 (0.96–1.95) | 0.08 |
| BMI | 1 kg/m2 | 1.08 (0.99–1.17) | 0.10 | 1.03 (0.97–1.08) | 0.33 |
| Systolic blood pressure | 1 mmHg | 1.02 (1.00–1.04) | 0.05 | 1.01 (1.00–1.02) | 0.25 |
| Diastolic blood pressure | 1 mmHg | 1.01 (0.98–1.04) | 0.72 | 0.98 (0.97–1.00) | 0.06 |
| Dialysis duration | 1 month | 1.00 (1.00–1.01) | 0.43 | 1.00 (0.99–1.00) | 0.47 |
| Dialysis duration | 2 year | 0.92 (0.76–1.12) | 0.42 | 0.96 (0.87–1.07) | 0.46 |
| spKt/V | 1 | 0.54 (0.12–2.45) | 0.43 | 0.85 (0.37–1.95) | 0.70 |
| Smoking history | Present vs. absent | 1.39 (0.74–2.61) | 0.30 | 0.88 (0.61–1.27) | 0.50 |
| Underlying kidney disease | |||||
| Primary hypertension | Present vs. absent | 1.56 (0.81–3.00) | 0.19 | 1.92 (1.37–2.75) | <0.001 |
| Coronary heart disease | Present vs. absent | 6.11 (2.77–13.48) | <0.001 | 3.27 (1.89–5.65) | <0.001 |
| Diabetes | Present vs. absent | 3.07 (1.49–6.30) | 0.002 | 2.17 (1.35–3.31) | 0.001 |
| Cerebral infarction | Present vs. absent | 2.34 (1.11–4.96) | 0.03 | 2.19 (1.43–3.37) | <0.001 |
| Cerebral hemorrhage | Present vs. absent | 2.76 (0.85–8.97) | 0.09 | 1.43 (0.59–3.50) | 0.43 |
| Gout | Present vs. absent | 0.41 (0.16–1.06) | 0.07 | 0.72 (0.46–1.11) | 0.17 |
| CCB | Yes vs. no | 0.69 (0.37–1.30) | 0.25 | 0.73 (0.51–1.04) | 0.09 |
| ACEI | Yes vs. no | 0.62 (0.24–1.60) | 0.33 | 0.73 (0.44–1.20) | 0.22 |
| ARB | Yes vs. no | 0.62 (0.29–1.35) | 0.23 | 0.69 (0.45–1.06) | 0.09 |
| β-Blocker | Yes vs. no | 1.52 (0.72–3.20) | 0.27 | 0.81 (0.49–1.36) | 0.43 |
| α-Blocker | Yes vs. no | 0.54 (0.19–1.51) | 0.24 | 1.03 (0.65–1.63) | 0.90 |
| Aspirin | Yes vs. no | 1.13 (0.53–2.37) | 0.76 | 0.97 (0.62–1.50) | 0.88 |
| Statin | Yes vs. no | 1.59 (0.49–5.16) | 0.44 | 1.16 (0.54–2.49) | 0.71 |
| Iron supplement | Yes vs. no | 0.59 (0.27–1.29) | 0.19 | 0.85 (0.57–1.26) | 0.41 |
| Calcium supplement | Yes vs. no | 0.41 (0.22–0.77) | 0.005 | 0.59 (0.41–0.84) | 0.004 |
| Active vitamin D | Yes vs. no | 0.38 (0.20–0.73) | 0.004 | 0.53 (0.37–0.76) | <0.001 |
| Albumin | 1 g/L | 0.90 (0.83–0.98) | 0.02 | 0.92 (0.88–0.97) | 0.001 |
| Prealbumin | 1 g/L | 0.00 (0.00–0.02) | <0.001 | 0.002 (0.00–0.02) | <0.001 |
| Hemoglobin | 1 g/L | 0.98 (0.96–1.00) | 0.09 | 0.99 (0.97–1.00) | 0.03 |
| BUN | 1 mmol/L | 1.02 (0.96–1.08) | 0.52 | 0.99 (0.96–1.03) | 0.70 |
| Creatinine | 1 μmol/L | 0.999 (0.998–1.000) | 0.22 | 0.999 (0.998–0.999) | <0.001 |
| Uric acid | 1 mmol/L | 1.000 (0.996–1.004) | 0.89 | 1.000 (0.997–1.001) | 0.44 |
| Calcium | 1 mmol/L | 0.69 (0.15–3.09) | 0.63 | 2.17 (0.91–5.19) | 0.08 |
| Phosphorus | 1 mmol/L | 1.07 (0.65–1.77) | 0.78 | 0.95 (0.72–1.26) | 0.71 |
| Alkaline phosphatase | 1 U/L | 1.000 (0.999–1.003) | 0.34 | 1.000 (1.000–1.002) | 0.02 |
| 25-Hydroxy vitamin D | 1 mmol/L | 1.00 (0.98–1.01) | 0.57 | 0.99 (0.98–1.01) | 0.55 |
| Iron | 1 μmol/L | 0.92 (0.86–0.99) | 0.03 | 0.96 (0.93–0.99) | 0.04 |
| UIBC | 1 μmol/L | 1.03 (0.99–1.06) | 0.12 | 1.01 (0.99–1.03) | 0.27 |
| TIBC | 1 μmol/L | 1.00 (0.96–1.05) | 0.95 | 0.99 (0.97–1.02) | 0.78 |
| Transferrin | 1 g/L | 1.22 (0.63–2.37) | 0.56 | 1.09 (0.73–1.60) | 0.68 |
| Ferritin | 1 ng/mL | 0.999 (0.998–1.001) | 0.57 | 0.999 (0.998–1.000) | 0.29 |
| iPTH | 1 pg/ml | 1.000 (1.000–1.001) | 0.28 | 1.000 (1.000–1.001) | 0.33 |
| hsCRP | 1 mg/L | 1.01 (1.00–1.02) | 0.04 | 1.01 (1.00–1.01) | 0.03 |
| Triglycerides | 1 mmol/L | 1.03 (0.89–1.19) | 0.68 | 1.02 (0.94–1.12) | 0.59 |
| Total cholesterol | 1 mmol/L | 0.89 (0.66–1.21) | 0.46 | 0.89 (0.75–1.05) | 0.16 |
| HDL-C | 1 mmol/L | 0.68 (0.25–1.81) | 0.44 | 0.85 (0.50–1.45) | 0.54 |
| LDL-C | 1 mmol/L | 0.85 (0.58–1.28) | 0.40 | 0.84 (0.68–1.04) | 0.11 |
| Apo-A | 1 mmol/L | 0.61 (0.17–2.22) | 0.45 | 0.73 (0.35–1.50) | 0.39 |
| Apo-B | 1 mmol/L | 0.36 (0.07–1.80) | 0.21 | 0.46 (0.19–1.13) | 0.09 |
| Lp (a) | 1 mmol/L | 1.000 (0.998–1.001) | 0.64 | 1.000 (0.999–1.001) | 0.91 |
| Homocysteine | 1 μmol/L | 1.00 (0.99–1.01) | 0.89 | 1.00 (0.99–1.00) | 0.55 |
| β2-Microglobulin | 1 mg/L | 1.00 (0.97–1.03) | 1.00 | 1.01 (0.99–1.02) | 0.58 |
| TMAO (Continuous variable) | 1 μg/mL | 1.13 (1.03–1.24) | 0.01 | 1.12 (1.07–1.18) | <0.001 |
| TMAO (dichotomous variable) | High vs. low | 2.52 (1.28–4.97) | 0.008 | 2.15 (1.48–3.12) | <0.001 |
*Variables with p < 0.1 for all-cause mortality and cardiovascular mortality are in bold.
Conversion of units: Transferrin: 1 g/L = 12.3 μmol/L; Ferritin: 1 ng/mL = 2.247 pmol/L; hsCRP: 1 mg/L = 0.9524 nmol/L; TMAO: 1 μg/mL = 13.3 μmol/L.
We then developed 8 different multivariate models that adjusted for different confounders to further examine the association of TMAO with cardiovascular death (Table 3) and all-cause death (Table 4). TMAO remained a significant risk factor for both endpoints in models 1–7.
Table 3.
Multivariate Cox proportional hazard model of cardiovascular death in HD patients in which TMAO was considered a continuous variable or a dichotomous variable.
| Model | Cardiovascular death |
|
|---|---|---|
| HR (95%CI) | p Value | |
| TMAO (continuous variable) | ||
| Unadjusted | 1.13 (1.03–1.24) | 0.01 |
| Model 1 | 1.13 (1.03–1.24) | 0.01 |
| Model 2 | 1.12 (1.01–1.25) | 0.03 |
| Model 3 | 1.12 (1.03–1.23) | 0.01 |
| Model 4 | 1.17 (1.06–1.28) | 0.001 |
| Model 5 | 1.13 (1.03–1.23) | 0.01 |
| Model 6 | 1.11 (1.01–1.22) | 0.03 |
| Model 7 | 1.13 (1.03–1.25) | 0.01 |
| Model 8 | 1.18 (1.07–1.29) | 0.001 |
| TMAO (dichotomous variable) | ||
| Unadjusted | 2.52 (1.28–4.97) | 0.008 |
| Model 1 | 2.52 (1.23–5.14) | 0.02 |
| Model 2 | 2.46 (1.20–5.04) | 0.01 |
| Model 3 | 2.66 (1.32–5.38) | 0.006 |
| Model 4 | 3.50 (1.65–7.43) | 0.001 |
| Model 5 | 2.59 (1.30–5.13) | 0.007 |
| Model 6 | 2.47 (1.24–4.94) | 0.01 |
| Model 7 | 2.68 (1.33–5.40) | 0.006 |
| Model 8 | 3.44 (1.67–7.08) | 0.001 |
Model 1: adjusted for age, gender, BMI, systolic blood pressure, diastolic blood pressure, dialysis duration, average interdialytic weight gain, residual renal function, and single-pool Kt/V.
Model 2: adjusted for smoking history, underlying kidney disease, history of primary hypertension, coronary heart disease, diabetes, cerebral infarction, cerebral hemorrhage, and gout.
Model 3: adjusted for history of taking CCB, ACEI, ARB, β-blocker, α-blocker, aspirin, statin, iron supplement, calcium supplement, and active vitamin D.
Model 4: adjusted for albumin, prealbumin, hemoglobin, BUN, creatinine, uric acid, β2-microglobulin, indoxyl sulfate, and hsCRP.
Model 5: adjusted for calcium, phosphorus, alkaline phosphatase, 25-hydroxy vitamin D, and iPTH;.
Model 6: adjusted for iron, UIBC, TIBC, transferrin, and ferritin.
Model 7: adjusted for triglycerides, total cholesterol, HDL-C, LDL-C, Apo-A, Apo-B, Lp (a), and homocysteine.
Model 8: adjusted for hierarchically selected covariates (p < 0.1 in the univariate Cox proportional hazard model): age, systolic blood pressure, coronary heart disease, diabetes, cerebral infarction, cerebral hemorrhage, gout, calcium supplement, active vitamin D, albumin, prealbumin, hemoglobin, iron, and hsCRP.
Table 4.
Multivariate Cox proportional hazard model of all-cause death in HD patients in which TMAO was considered as a continuous variable or a dichotomous variable.
| Model | All-cause death |
|
|---|---|---|
| HR (95%CI) | p Value | |
| TMAO (continuous variable) | ||
| Unadjusted | 1.12 (1.07–1.18) | <0.001 |
| Model 1 | 1.12 (1.07–1.18) | <0.001 |
| Model 2 | 1.10 (1.04–1.16) | 0.001 |
| Model 3 | 1.12 (1.06–1.18) | <0.001 |
| Model 4 | 1.15 (1.09–1.21) | <0.001 |
| Model 5 | 1.13 (1.08–1.19) | <0.001 |
| Model 6 | 1.12 (1.06–1.18) | <0.001 |
| Model 7 | 1.14 (1.08–1.20) | <0.001 |
| Model 8 | 1.14 (1.08–1.21) | <0.001 |
| TMAO (dichotomous variable) | ||
| Unadjusted | 2.15 (1.48–3.12) | <0.001 |
| Model 1 | 2.11 (1.42–3.12) | <0.001 |
| Model 2 | 2.18 (1.49–3.20) | <0.001 |
| Model 3 | 2.09 (1.43–3.07) | <0.001 |
| Model 4 | 2.75 (1.84–4.11) | <0.001 |
| Model 5 | 2.26 (1.55–3.30) | <0.001 |
| Model 6 | 2.16 (1.47–3.15) | <0.001 |
| Model 7 | 2.35 (1.60–3.46) | <0.001 |
| Model 8 | 2.54 (1.71–3.76) | <0.001 |
Model 1: adjusted for age, gender, BMI, systolic blood pressure, diastolic blood pressure, dialysis duration, average interdialytic weight gain, residual renal function, and single-pool Kt/V.
Model 2: adjusted for smoking history, underlying kidney disease, history of primary hypertension, coronary heart disease, diabetes, cerebral infarction, cerebral hemorrhage, and gout.
Model 3: adjusted for history of taking CCB, ACEI, ARB, β-blocker, α-blocker, aspirin, statin, iron supplement, calcium supplement, and active vitamin D.
Model 4: adjusted for albumin, prealbumin, hemoglobin, BUN, creatinine, uric acid, β2-microglobulin, indoxyl sulfate, and hsCRP.
Model 5: adjusted for calcium, phosphorus, alkaline phosphatase, 25-hydroxy vitamin D and iPTH.
Model 6: adjusted for iron, UIBC, TIBC, transferrin, and ferritin.
Model 7: adjusted for triglycerides, total cholesterol, HDL-C, LDL-C, Apo-A, Apo-B, Lp (a), and homocysteine.
Model 8: adjusted for hierarchically selected covariates (p < 0.1 in the univariate Cox proportional hazard model): age, gender, diastolic blood pressure, primary hypertension, coronary heart disease, diabetes, cerebral infarction, history of taking medicine (including CCB, ARB, calcium supplement, active vitamin D), albumin, prealbumin, hemoglobin, creatinine, calcium, alkaline phosphatase, iron, hsCRP, and Apo-B.
We used hierarchical selection procedures to establish model 8, in which the inclusion criterion for model selection in the covariate set was predetermined as a p-value below 0.10 in the univariate Cox proportional hazard model. Thus, the covariates of model 8 for cardiovascular death were age, systolic blood pressure, history of coronary heart disease, history of diabetes, history of cerebral infarction, history of cerebral hemorrhage, history of gout, taking calcium supplement and active vitamin D, albumin, prealbumin, hemoglobin, and hsCRP. The results showed that TMAO was significantly associated with cardiovascular death when it was treated as a continuous variable (HR: 1.18, 95% CI: 1.07, 1.29, p = 0.001) or dichotomous variable (HR: 3.44, 95% CI: 1.67, 7.08, p = 0.001) (Table 3).
The covariates of model 8 for all-cause death were age, gender, diastolic blood pressure, history of primary hypertension, history of coronary heart disease, history of diabetes, history of cerebral infarction, history of taking medicine (angiotensin II receptor blocker [ARB], calcium supplement and active vitamin D), albumin, prealbumin, hemoglobin, creatinine, plasma calcium, alkaline phosphatase, iron, hsCRP, and apoprotein-B. The results showed that TMAO remained significantly associated with all-cause death when it was treated as a continuous variable (HR: 1.14, 95% CI: 1.08, 1.21, p < 0.001) or a dichotomous variable (HR: 2.54, 95% CI: 1.71, 3.76, p < 0.001) (Table 4). Eliminated patients with DM, we displayed all the models and found that high plasma TMAO level is still significantly associated with cardiovascular and all-cause mortality in HD patients (Supplemental Tables 3 and 4). The high and low TMAO groups had no significant difference in cerebrovascular death (p = 0.23).
Discussion
This prospective cohort study of patients who were receiving HD due to minimal or no residual renal function indicated the presence of significant and independent associations of plasma TMAO level with cardiovascular and all-cause mortality. The mean TMAO concentration in our cohort was 5.29 ± 2.97 μg/mL (70.5 ± 39.6 μmol/L), more than 20-times higher than previously reported for individuals with normal renal function [18]. Our analysis of cardiovascular mortality indicated the high-TMAO group had an 18% higher risk (with TMAO as a continuous variable) or 244% higher risk (with TMAO as a dichotomous variable). Our analysis of all-cause mortality indicated the high-TMAO group had a 14% higher risk (with TMAO as a continuous variable) or a 154% higher risk (with TMAO as a dichotomous variable). These findings strongly suggest that an elevated TMAO level is directly responsible for vascular toxicity in HD patients.
The identities of the uremic toxins responsible for the increased morbidity and mortality in patients undergoing HD are largely unknown [23]. Consequently, no known treatment regimens can remove the responsible toxins and thereby reduce the acceleration of cardiovascular disease in these patients [23]. Although most patients receive ‘adequate’ dialysis based on urea clearance (Kt/VUREA), their quality of life and outcomes remain poor [24]. Some researchers hypothesized that increasing the dialysis dose and flux would clear more uremic toxins and improve the outcomes of these patients. However, the HEMO study showed that high-dose dialysis, which provided a 30% increase in urea clearance (Kt/VUREA), did not improve patient survival [25]. Because the identities of the specific uremic toxins are unknown, it is also challenging to implement individualized dialysis treatments [23]. Our study identified TMAO as a modifiable active molecule that is potentially responsible for cardiovascular toxicity in HD patients.
Plasma TMAO levels in HD patients reflect the net effect of TMAO production and clearance [18]. Interactions of the diet, gut microbiota, and host are responsible for TMAO production [8,26]. In particular, gut microbiota can lyse various trimethylamine (TMA) -containing compounds to produce free TMA. TMA then passes into the blood through the intestine, and hepatic flavin monooxygenase 3 produces TMAO, which has potent biological activity [8,27]. Intestinal microbiota produce TMA from diverse precursor molecules, such as TMAO, choline, phosphatidylcholine, carnitine, and betaine, all of which are common in animal diets [26]. In healthy individuals with normal renal function, TMAO is not protein-bound, and is rapidly cleared from circulation by the kidneys. Soon after an HD session, the plasma TMAO levels of HD patients are similar to those with normal renal function [28]; however, HD patients rapidly accumulate TMAO between dialysis sessions due to decreased excretion. The predialysis plasma TMAO levels of HD patients are more than 20 times higher than those of individuals with normal renal function [18]. High TMAO concentration in animal models leads to increased expression of the scavenger receptors CD36 and SR-A1 and increased uptake of modified low-density lipoproteins into foam cells by macrophages [8]. By contrast, a low circulating TMAO level (mediated by inhibition of choline TMA lyase) attenuated the promoting role of choline in atherosclerosis [29]. In agreement, patients with CKD who have elevated plasma TMAO levels have poor cardiovascular outcomes [15–17]. Our findings extend this connection to patients undergoing HD.
The levels of p-cresol sulfate, indoxyl sulfate, and TMAO are all associated with adverse outcomes in HD patients [5,21,30], but their underlying mechanisms differ. P-cresol sulfate mainly mediates vascular toxicity by endothelial dysfunction and leukocyte activation [31]; indoxyl reportedly causes myocardial hypertrophy and myocardial fibrosis [31,32]; and TMAO mainly promotes atherosclerosis and increases the risk of thrombosis [10,27].
It is important to compare our findings with those of three prior publications that also investigated the relationship of TMAO with cardiovascular and all-cause mortality in HD patients. Kaysen et al. [19] investigated the association of serum TMAO with cardiovascular and all-cause mortality in 235 incident HD patients in the Comprehensive Dialysis Study (CDS). Their median TMAO concentration (43 μmol/L) was about 30% lower than in our patients (63.1 μmol/L), although still markedly higher than in patients with normal renal function. These researchers found no association of TMAO with cardiovascular or all-cause mortality. However, they assessed all-cause mortality based on claims data, which were only available for 152 patients (65%). Besides, their endpoint events were not diagnosed and recorded directly by physicians, and the lack of follow-up data limited their ability to investigate the association of TMAO with cardiovascular or all-cause mortality. Stubbs et al. [20] also reported no association of TMAO with cardiovascular or all-cause mortality in 1243 participants in the control arm of the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial. However, all of these patients had moderate to severe secondary hyperparathyroidism, and about 80% of them had TMAO concentrations of 56 μmol/L or more (similar to the median of our study, 63.1 μmol/L). Although the endpoint events of this study were approximately 35%, the cardiovascular damage caused by elevated TMAO concentrations was extremely high due to the co-occurrence of moderate to severe hyperparathyroidism and the high TMAO load, making it difficult to assess the independent association of cardiovascular damage with elevated TMAO concentration [33]. According to the diagnostic criteria used for moderate to severe hyperparathyroidism in this previous study [34], our study had 72 patients (28.6%) with moderate to severe hyperparathyroidism, 33 patients (26.2%) in the low-TMAO group and 39 patients (31.0%) in the high-TMAO group (p = 0.403).
A recent cohort study by Zheng et al. [35] found that TMAO was an independent risk factor for hospitalization in HD patients, and another study reported that TMAO was associated with a higher mortality rate after hospitalization of HD patients [36]. This result supports our conclusion that a high plasma TMAO level is significantly and independently associated with all-cause mortality in HD patients.
Shafi et al. [21] examined samples from 1232 HD patients in the HEMO study and reported that cardiovascular morbidity and mortality were more significant in those with higher TMAO levels, especially among those with white ethnicity. Their median TMAO concentration (88 μmol/L) was about 40% higher than in our patients (63.1 μmol/L). However, this previous study did not provide data on CRP and did not adjust for the confounding effect of inflammation on outcomes. Inflammation has a crucial role in the development of atherosclerosis [37–39], and reducing the CRP level can reduce the incidence of major cardiovascular events [40]. Chronic inflammation is characterized by an elevated CRP level and is also an independent predictor of cardiovascular and all-cause mortality in HD patients [41–43]. In contrast, we found that after adjustment for CRP (model 4), an elevated TMAO level remained associated with cardiovascular and all-cause mortality. Thus, our study provides more reliable evidence that plasma TMAO concentration is directly associated with cardiovascular and all-cause mortality in HD patients.
One limitation of the present study is that TMAO was measured only once, and changes in renal function, intestinal microbiota, and diet during the study may have affected TMAO concentrations. A second limitation is that this was a single-center study. Future studies that record TMAO levels in HD patients at multiple times and multiple centers may help to verify the potential utility of clinical interventions that target TMAO.
In conclusion, TMAO is associated with cardiovascular and all-cause mortality in HD patients. Our findings call for a carefully designed clinical trial to determine the effect of lowering TMAO concentrations on cardiovascular outcomes in HD patients.
Supplementary Material
Acknowledgments
The authors would like to thank all the staff at the Blood Purification Center, Zhongshan Hospital, Fudan University.
Funding Statement
This study was supported by the National Key Research and Development Program [No. 2016YFC1305500], the National Natural Scientific Foundation of China [No. 91849123], and Shanghai Municipal Health Commission [No. 2017ZZ01015].
Disclosure statement
The results presented in this paper have not been published previously in whole or part, except an abstract of this manuscript was presented earlier in ASN’s Kidney Week 2019 in Washington, DC (NO. 3236604).
References
- 1.United States Renal Data System . 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 2.Himmelfarb J, Ikizler TA.. Hemodialysis. N Engl J Med. 2010;363(19):1833–1845. [DOI] [PubMed] [Google Scholar]
- 3.Longenecker JC, Coresh J, Powe NR, et al. . Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13(7):1918–1927. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson JAP, Wheeler DC.. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int. 2017;92(4):809–815. [DOI] [PubMed] [Google Scholar]
- 5.Cao XS, Chen J, Zou JZ, et al. . Association of indoxyl sulfate with heart failure among patients on hemodialysis. CJASN. 2015;10(1):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijers BK, Bammens B, De Moor B, et al. . Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008;73(10):1174–1180. [DOI] [PubMed] [Google Scholar]
- 7.Meyer TW, Hostetter TH.. Approaches to Uremia. J Am Soc Nephrol. 2014;25(10):2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, et al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WH, Wang Z, Levison BS, et al. . Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Gregory JC, Org E, et al. . Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seldin MM, Meng Y, Qi H, et al. . Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc. 2016;5(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun XL, Jiao XF, Ma YR, et al. . Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481(1–2):63–70. [DOI] [PubMed] [Google Scholar]
- 13.Chen ML, Zhu XH, Ran L, et al. . Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6(9):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Henderson A, Petriello MC, et al. . Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30(6):1141–1151.e1145. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs JR, House JA, Ocque AJ, et al. . Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. JASN. 2016;27(1):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim RB, Morse BL, Djurdjev O, et al. . Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89(5):1144–1152. [DOI] [PubMed] [Google Scholar]
- 17.Tang WH, Wang Z, Kennedy DJ, et al. . Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hai X, Landeras V, Dobre MA, et al. . Mechanism of prominent trimethylamine oxide (TMAO) accumulation in hemodialysis patients. PLoS One. 2015;10(12):e0143731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaysen GA, Johansen KL, Chertow GM, et al. . Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25(4):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stubbs JR, Stedman MR, Liu S, et al. . Trimethylamine N-oxide and cardiovascular outcomes in patients with ESKD receiving maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14(2):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafi T, Powe NR, Meyer TW, et al. . Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol. 2017;28(1):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Depner TA, Daugirdas JT.. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol. 1996;7(5):780–785. [DOI] [PubMed] [Google Scholar]
- 23.Meyer TW, Sirich TL, Hostetter TH.. Dialysis cannot be dosed. Semin Dial. 2011;24(5):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Depner TA. Uremic toxicity: urea and beyond. Semin Dial. 2001;14(4):246–251. [DOI] [PubMed] [Google Scholar]
- 25.Eknoyan G, Beck GJ, Cheung AK, Hemodialysis (HEMO) Study Group, et al.. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZN, Zhao YZ.. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9(5):416–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koeth RA, Wang ZE, Levison BS, et al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bain MA, Faull R, Fornasini G, et al. . Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21(5):1300–1304. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Roberts AB, Buffa JA, et al. . Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu IW, Hsu KH, Hsu HJ, et al. . Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients-a prospective cohort study. Nephrol Dial Transplant. 2012;27(3):1169–1175. [DOI] [PubMed] [Google Scholar]
- 31.Vanholder R, Schepers E, Pletinck A, et al. . The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekawanvijit S, Adrahtas A, Kelly DJ, et al. . Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31(14):1771–1779. [DOI] [PubMed] [Google Scholar]
- 33.Flythe JE, Hostetter TH.. Assessing clinical relevance of uremic toxins. Clin J Am Soc Nephrol. 2019;14(2):182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chertow GM, Block GA, Correa-Rotter R, et al. . Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Tang Z, You L, et al. . Trimethylamine-N-oxide is an independent risk factor for hospitalization events in patients receiving maintenance hemodialysis. Ren Fail. 2020;42(1):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu S, Fukuma S, Ikenoue T, et al. . Increased mortality rate after hospitalization among chronic hemodialysis patients: a prospective cohort study. Nephron. 2018;140(3):194–202. [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. [DOI] [PubMed] [Google Scholar]
- 38.Libby P, Ridker PM, Hansson GK,. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM, Everett BM, Pradhan A, et al. . Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, MacFadyen JG, Everett BM, et al. . Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann J, Herrlinger S, Pruy A, et al. . Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658. [DOI] [PubMed] [Google Scholar]
- 42.Yeun JY, Levine RA, Mantadilok V, et al. . C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35(3):469–476. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi AR, Alvestrand A, Divino JC, et al. . Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13(1):S28–S36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.