Figure 1.
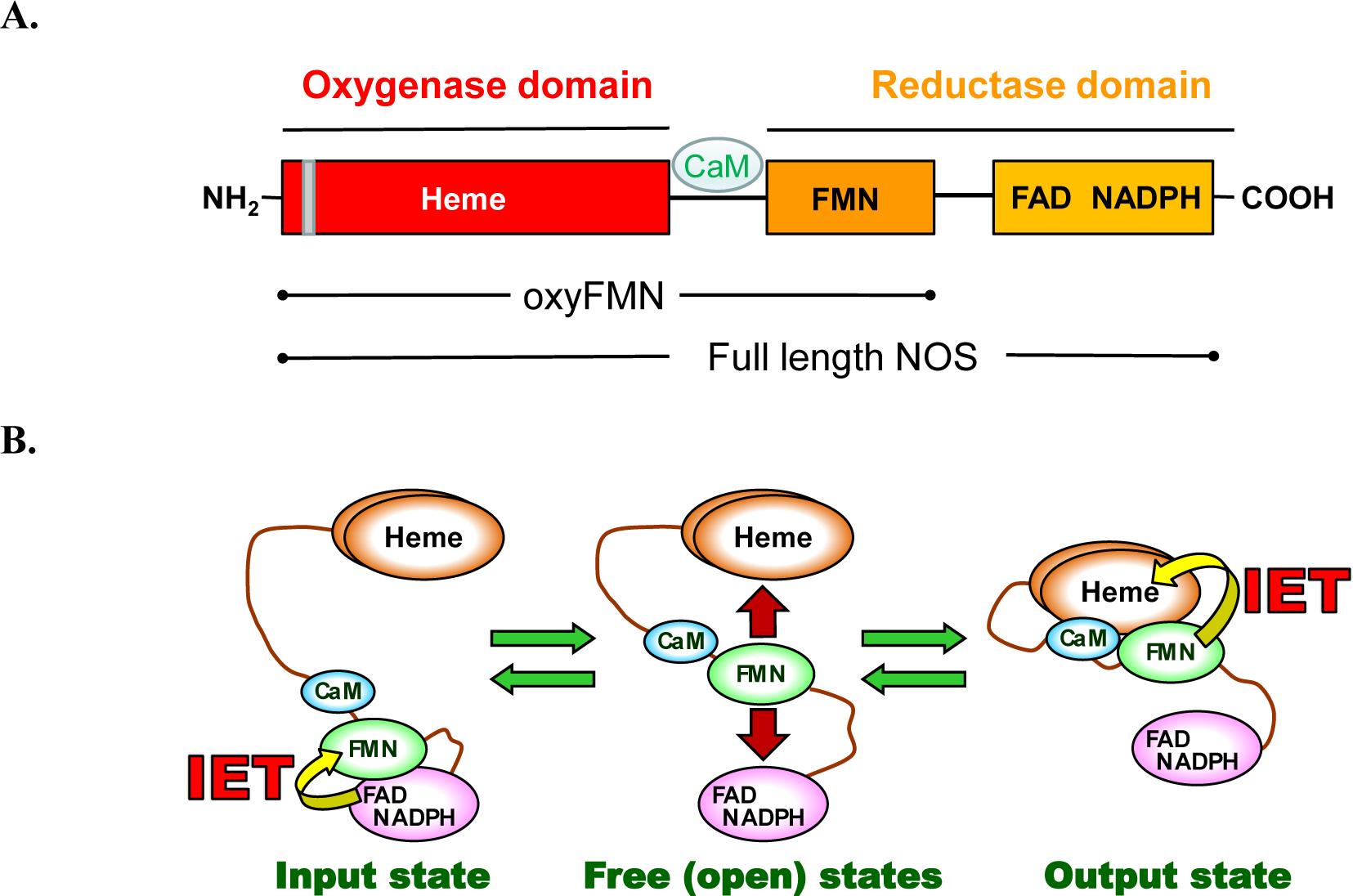
A. Domain organization of full-length and bi-domain oxyFMN iNOS proteins. The grey bar indicates the dimer interface in the heme-containing oxygenase domain. The oxyFMN and holo iNOS proteins used in this study are purified as dimers. B. Tether shuttle model for the NOS electron transport pathway. The FMN domain swings between the input and output states through the free/open states to transport the NADPH-derived electrons across the NOS domains.
