Abstract
Background
An association between inflammatory bowel disease (IBD) and obesity has been observed. Little is known about the effect of weight loss on IBD course. Our aim was to determine the impact of bariatric surgery on long-term clinical course of obese patients with IBD, either Crohn's disease (CD) or ulcerative colitis (UC).
Methods
Patients with IBD who underwent bariatric surgery subsequent to IBD diagnosis were identified from 2 tertiary IBD centers. Complications after bariatric surgery were recorded. Patients were matched 1:1 for age, sex, IBD subtype, phenotype, and location to patients with IBD who did not undergo bariatric surgery. Controls started follow-up at a time point in their disease similar to the disease duration in the matched case at the time of bariatric surgery. Inflammatory bowel disease medication usage and disease-related complications (need for corticosteroids, hospitalizations, and surgeries) among cases and controls were compared.
Results
Forty-seven patients met inclusion criteria. Appropriate matches were found for 25 cases. Median follow-up among cases (after bariatric surgery) and controls was 7.69 and 7.89 years, respectively. Median decrease in body mass index after bariatric surgery was 12.2. Rescue corticosteroid usage and IBD-related surgeries were numerically less common in cases than controls (24% vs 52%; odds ratio [OR], 0.36; 95% confidence interval [CI], 0.08–1.23; 12% vs 28%; OR, 0.2; 95% CI, 0.004–1.79). Two cases and 1 control were able to discontinue biologics during follow-up.
Conclusions
Inflammatory bowel disease patients with weight loss after bariatric surgery had fewer IBD-related complications compared with matched controls. This observation requires validation in a prospective study design.
Keywords: Bariatric surgery, Crohn's disease, ulcerative colitis, obesity
We matched 1:1 on 5 variables, 25 patients with inflammatory bowel disease (IBD) who underwent bariatric surgery matched to 25 controls who did not. Rescue corticosteroid usage and IBD-related surgeries were numerically less common in cases, whereas 2 cases and 1 control were able to discontinue biologics during follow-up.
INTRODUCTION
Obesity is a major health problem worldwide and is associated with a high health care burden.1 The prevalence of obesity in adults in the United States is ~39.8%, and its incidence continues to rise.2, 3 In parallel, the number of bariatric surgeries performed is also increasing and reached 216,000 procedures in 2016.4
Although early-onset inflammatory bowel disease (IBD) can lead to malnutrition, with failure to thrive as a presenting sign in >40% of patients,5 the prevalence of obesity in adult IBD patients is approximately 20%–40%.6, 7 Inflammatory bowel disease patients with obesity, compared with those without obesity, have more extra-intestinal manifestations and spend more days in the hospital annually, with higher hospitalization-related costs.8, 9 Additionally, obesity may result in suboptimal response to biologic therapy, potentially by promoting rapid clearance of biologic agents, leading to low trough concentrations.10 Obesity has also been shown to adversely impact abdominal surgery in IBD patients, both from the viewpoint of postoperative complications and in terms of technical challenges in constructing ileal pouch–anal anastomosis and stomas.10–13 Hence, obesity may be an adjunctive target in the management of IBD.10
Bariatric surgery has been shown to have an acceptable safety profile as a treatment option for obesity in this population.14–16 The impact of weight loss after bariatric surgery on the long-term disease course of IBD, however, remains largely unknown, although small case series have suggested that most patients' IBD did not worsen clinically.15, 17
The aims of this study were to evaluate the impact of weight loss achieved after bariatric surgery on the long-term IBD disease course and to report the safety of bariatric surgery on IBD patients from 2 large tertiary referral centers in the United States. We also performed a review of literature to report prior case series evaluating the efficacy and safety of bariatric surgery in IBD patients.
METHODS
Case Series
Electronic medical records from 2 large tertiary referral centers for IBD (Mayo Clinic, Rochester, MN, USA, and Washington University School of Medicine, St. Louis, MO, USA) were reviewed using informatics search tools to identify patients with co-occurrence of an ICD-9/10 code for Crohn's disease (CD) or ulcerative colitis (UC) and clinical note terms associated with bariatric surgery (Roux-en-Y, bariatric surgery, gastroplasty, gastric bypass, gastric sleeve, duodenal switch, gastric banding). The institutional review boards of both institutions approved the study. Medical records between January 1, 1996, and December 31, 2016, were reviewed. In addition, prior reports (full manuscript or abstract) on bariatric surgery in patients with IBD were identified on PUBMED using the MESH terms “inflammatory bowel diseases” and “bariatric surgery” separated by the Boolean operator “AND” from 1946 to present. The reference lists of identified articles were hand-searched to identify other case reports.
Demographic and disease characteristics, including previous medications and disease complications, were extracted from the medical record. The Montreal classification was used to classify disease extent in UC patients and disease extent and phenotype in patients with Crohn's disease.18 We captured the following complications within 90 days after bariatric surgery: readmission within 90 days, re-operation, anastomotic leak, reversal, incisional hernia, small bowel obstruction, ulcer, and stricture.
Case–Control
Patients with CD or UC were reviewed for potential inclusion as cases. Those with <1 year of follow-up were excluded. Ulcerative colitis patients with a history of colectomy before gastric bypass were also excluded from the case–control analysis but were analyzed for safety outcomes. Patients with IBD and without prior history of bariatric surgery were identified through medical indexing and matched 1:1 for age, sex, IBD subtype, and IBD diagnosis date. Crohn's disease patients were additionally matched on disease location (isolated ileal, ileocolonic, or isolated colonic) and phenotype (inflammatory, stricturing, or penetrating). Controls started follow-up at a time point in their disease similar to the disease duration in the matched cases at the time of bariatric surgery.
We recorded the median body mass index (BMI) in cases and controls and the change in BMI in cases. We captured the number of cases and controls who had an IBD complication, which included corticosteroids for active disease, IBD hospitalization, or IBD-related surgery. For ulcerative colitis, progression of disease was defined as progressing from ulcerative proctitis to left-sided or pancolitis or progression from left-sided to pancolitis. For Crohn's disease, progression of disease phenotype included progressing from an inflammatory phenotype to a stricturing or fistulizing phenotype or progressing from isolated ileal disease or isolated colonic disease to ileocolonic.
Statistical Analysis
The Wilcoxon signed rank test was used to compare median BMIs in cases and controls. McNemar's test was used to compare the number of IBD complications between cases and matched controls given the nonparametric distribution of data and limited number of outcomes.
RESULTS
Baseline Characteristics
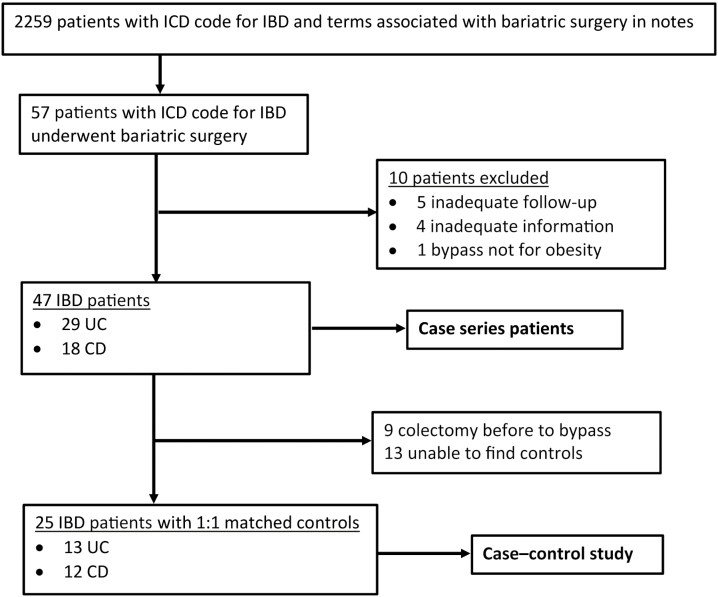
A total of 57 patients were identified by the initial search as having IBD and undergoing bariatric surgery (Fig. 1). After applying the exclusion criteria, 47 patients remained, 29 with UC and 18 with CD. We excluded 9 patients with UC from the case–control analysis because they had a colectomy before bariatric surgery. We were unable to find controls matched on all the specified criteria for 13 IBD patients, leaving 25 patients for the case–control portion of the analysis.
Figure 1.
Flowchart depicting case selection.
The baseline characteristics across 47 IBD patients are shown in Table 1. The median age at IBD diagnosis (interquartile range [IQR]) was 32 (25–38.5) years, whereas the median age at the time of bariatric surgery (IQR) was 49 (39–52.5) years. Most patients were female (83.0%), and the median BMI (IQR) was 46.6 (42.2–53.2). Roux-en-Y gastric bypass surgery was the most common weight loss procedure performed. Most patients with UC had extensive colitis. Penetrating and inflammatory were the most common CD phenotypes, and 38.9% of CD patients had a history of perianal disease. Most CD patients had both ileal and colonic involvement.
Table 1.
Baseline Characteristics of Patients With Inflammatory Bowel Disease who Underwent Bariatric Surgery
| Ulcerative Colitis (n = 29) | Crohn's Disease (n = 18) | |
|---|---|---|
| Age at bypass, median (IQR), y | 48 (39–51) | 50 (38.75–55.75) |
| Age at IBD diagnosis, median (IQR), y | 32 (23–39) | 34 (26.3–39.5) |
| Female sex, No. (%) | 21 (72.4) | 18 (100.0) |
| BMI at time of bypass, median (IQR), kg/m2 | 44.2 (41.6–51.3) | 47.15 (43.5–54.3) |
| % EWL, mean (SD) | 37.9 (21.2) | 50.4 (31) |
| Weight loss, mean (SD), lb | 55.8 (35.1) | 73.9 (40.5) |
| Type of bariatric procedure, No. (%) | ||
| Roux-en-Y | 16 (55.2) | 12 (66.7) |
| Sleeve | 5 (17.2) | 3 (16.7) |
| Stapling | - | 1 (5.6) |
| Gastric banding | 6 (20.7) | 2 (11.1) |
| Othera | 2 (6.9) | - |
| Disease location (CD), No. (%) | ||
| L1, ileal | 3 (16.7) | |
| L2, colonic | 3 (16.7) | |
| L3, ileocolonic | 12 (66.7) | |
| L4, upper gut involvement | 4 (22.2) | |
| History of perianal disease, No. (%) | 7 (38.9) | |
| Disease behavior (CD), No. (%) | ||
| B1, nonstricturing, nonpenetrating | 8 (44.4) | |
| B2, stricturing | 3 (16.7) | |
| B3, penetrating | 7 (38.9) | |
| Disease location (UC), No. (%)b,c | ||
| Proctitis | 2 (10.5) | |
| Left-sided colitis | 6 (31.6) | |
| Extensive colitis | 11 (57.9) | |
| Medications before surgery, No. (%)b | ||
| Aminosalicylate | 10 (50.0) | 2 (12.5) |
| Thiopurine | 4 (20.0) | 5 (31.3) |
| Methotrexate | - | 1 (6.3) |
| TNF-α inhibitor | 2 (10.0) | 1 (6.3) |
| TNF-α inhibitor + thiopurine | 2 (10.0) | 4 (25.0) |
| Corticosteroidsd | 2 (10.0) | 3 (18.8) |
| Need for systemic steroids, ever, No. (%) | 18 (62.1) | 13 (72.2) |
| Need for IBD-related hospitalization, No. (%) | 16 (55.2) | 13 (72.2) |
| Need for IBD-related surgery, No. (%) | 9 (31.0) | 7 (38.9) |
| Smoking, No. (%) | ||
| Current | 1 (3.4) | 2 (11.1) |
| Never | 21 (72.4) | 7 (38.9) |
| Former | 7 (24.1) | 9 (50.0) |
| Endoscopic severity, No. (%) be | ||
| Inactive | 7 (70.0) | 5 (45.5) |
| Mild | 3 (30.0) | 5 (45.5) |
| Moderate | 0 | 1 (9.1) |
| Severe | 0 | 0 |
aOne patient had jejunoileal bypass, and 1 patient had Billroth II with near total gastric exclusion.
bExcluding 9 UC patients who had a colectomy before bariatric surgery.
cOne patient with UC did not have disease extent recorded.
dThree patients with UC and 1 patient with CD were on prednisone and an aminosalicylate. One patient with CD was on budesonide and a thiopurine. Medication information missing for 2 CD patients.
eNo endoscopic information was available for 10 patients with UC and 7 patients with CD.
The baseline characteristics across the 25 IBD cases and their matched controls are shown in Table 2. Among cases, Roux-en-Y (60%) and gastric banding (24%) were the 2 most common types of bariatric surgery. Among cases, weight loss was recorded after bariatric surgery among patients with CD (median body mass index [BMI], 49.5 before vs 34 after) and UC (median BMI, 43 before vs 32.8 after). Among UC cases, extensive colitis was the predominant disease extent before bariatric surgery (61.5%). In CD cases, the most common disease extent before bariatric surgery was ileocolonic (79%), whereas penetrating phenotype (41.2%) was the predominant disease behavior (Table 2).
Table 2.
Baseline Characteristics of Patients With Inflammatory Bowel Disease who Underwent Bariatric Surgery and Matched Controls
| Cases | Controls | |||
|---|---|---|---|---|
| Ulcerative Colitis (n = 13) | Crohn's Disease (n = 12) | Ulcerative Colitis (n = 13) | Crohn's Disease (n = 12) | |
| Age at bypass, median (IQR), y | 41 (39–49.5) | 44.5 (37.2–51.7) | - | - |
| Age at IBD diagnosis, median (IQR), y | 32 (18.5–38.5) | 33 (25–35) | 36 (25–43) | 29.5 (22.7–38.5) |
| Female sex, No. (%) | 11 (84.6) | 12 (100) | 11 (84.6) | 12 (100) |
| BMI at time of bypass, median (IQR), kg/m2 | 43 (41.6–49) | 49.5 (42–56.4) | - | - |
| BMI 1 y after bypass, median (IQR), kg/m2 | 36.2 (28.7–39.9) | 31.8 (28.9–37.9) | - | - |
| BMI current, median (IQR), kg/m2 | 32.8 (31.4–39.5) | 34 (28–44.5) | 26.6 (21.9–38.9) | 23.8 (21.8–29.8) |
| Weight at time of bypass, median (IQR), lb | 121.1 (98.2–141.5) | 125.5 (105.1–159.1) | ||
| Weight at 1 y after bypass, median (IQR), lb | 100 (73–117.7) | 78.2 (69.5–109.3) | ||
| Weight current, median (IQR), lb | 90 (19.3–127.1) | 80.2 (71.3–117.3) | ||
| TWL, mean (SD), kg | 24.58 (15.23) | 41.87 (15.9) | - | - |
| % TWL, mean (SD) | 18.62 (12.81) | 31.31 (15.99) | - | - |
| Type of bariatric procedure, No. (%) | ||||
| Roux-en-Y | 6 (46.1) | 9 (75.0) | - | - |
| Sleeve | 2 (15.4) | 0 (0) | - | - |
| Stapling | 0 | 1 (8.3) | - | - |
| Gastric banding | 4 (30.7) | 2 (16.6) | - | - |
| Jejunoileal bypass | 1 (7.7) | 0 | - | - |
| Disease location (CD), No. (%) | ||||
| L1, ileal | - | 2 (16.6) | - | 2 (16.6) |
| L2, colonic | - | 1 (8.3) | - | 1 (8.3) |
| L3, ileocolonic | - | 9 (75.0) | - | 9 (75.0) |
| History of perianal disease, No. (%) | 5 (41.6) | - | 4 (33.3) | |
| Disease behavior (CD), No. (%) | ||||
| B1, nonstricturing, nonpenetrating | - | 5 (41.6) | - | 5 (41.6) |
| B2, stricturing | - | 1 (8.3) | - | 1 (8.3) |
| B3, penetrating | - | 6 (50.0) | - | 6 (50.0) |
| Disease location (UC), No. (%) | ||||
| Proctitis | 1 (7.7) | - | 1 (7.7) | - |
| Left-sided colitis | 4 (30.7) | - | 4 (30.7) | - |
| Extensive colitis | 8 (61.5) | - | 8 (61.5) | - |
| Medications before surgery, No. (%) | ||||
| Aminosalicylate | 6 (46.1) | 2 (16.6) | 6 (46.1) | 3 (25.0) |
| Immune modulator monotherapy | 4 (30.7) | 5 (41.6) | 4 (30.7) | 3 (25.0) |
| Biologics | 2 (15.4) | 4 (33.3) | 2 (15.4) | 6 (50.0) |
| Need for systemic steroids, ever, No. (%) | 6 (46.1) | 9 (75.0) | 10 (76.9) | 10 (83.3) |
| Need for IBD-related hospitalization, No. (%) | 5 (38.4) | 9 (75.0) | 3 (23.1) | 8 (66.6) |
| Need for IBD-related surgery, No. (%) | 0 (0) | 4 (33.3) | 0 (0) | 7 (58.3) |
| Smoking, No. (%) | ||||
| Current | 0 (0) | 2 (16.6) | 0 (0) | 4 (33.3) |
| Never | 12 (92.3) | 5 (41.6) | 6 (46.1) | 4 (33.3) |
| Former | 1 (7.7) | 5 (41.6) | 7 (53.8) | 4 (33.3) |
| Endoscopic severity, No. (%) | ||||
| Inactive | 7 (53.8) | 4 (33.3) | 0 (0) | 1 (8.3) |
| Mild | 3 (23.1) | 4 (33.3) | 3 (23.1) | 3 (25.0) |
| Moderate | 0 (0) | 1 (8.3) | 6 (46.1) | 1 (8.3) |
| Severe | 0 (0) | 0 (0) | 2 (15.4) | 0 (0) |
Impact of Weight Loss After Bariatric Surgery on IBD
Median follow-up of cases (after bariatric surgery) and controls was 7.69 and 7.89 years, respectively. Inflammatory bowel disease patients had a decrease in median BMI of 12.2 kg/m2 after bariatric surgery across the 25 patients, but the BMI at last recorded follow-up remained greater than the BMI of control patients (Table 3). Overall, IBD-related outcomes were numerically less common in cases than controls (48% vs 72%, respectively; odds ratio [OR], 0.44; 95% confidence interval [CI], 0.1–1.60; P = 0.27), specifically in rescue corticosteroid use and IBD-related surgeries. A similar number of cases and controls required hospitalizations during the period of follow-up.
Table 3.
Inflammatory Bowel Disease Course After Bariatric Surgery in Cases Compared With Matched Controls
| IBD (n = 25) | Controls (n = 25) | P | ||
|---|---|---|---|---|
| BMI at last follow-up, median (IQR), kg/m2 | 33.8 (31.1–40.1) | 25.0 (21.8–32.3) | <0.01 | |
| Change in BMI, median (IQR), kg/m2 | 12.2 (6.6–14.4) | |||
| Continuing on a biologic, No. (%) | 4 (16) | 7 (28) | 0.25 | |
| Need for a new biologic, No. (%) | 4 (16) | 6 (24) | 0.5 | |
| Odds Ratio | P | |||
| Any IBD complication, No. (%) | 12 (48.0) | 18 (72.0) | 0.44 (0.1–1.60) | 0.27 |
| Corticosteroids for active disease, No. (%) | 6 (24.0) | 13 (52.0) | 0.36 (0.08–1.23) | 0.12 |
| IBD hospitalization, No. (%) | 9 (36.0) | 9 (36.0) | 1 (0.19–5.4) | 0.72 |
| IBD-associated surgery, No. (%) | 3 (12) | 7 (28.0) | 0.2 (0.004–1.79) | 0.22 |
Among cases, 1 patient with CD progressed from inflammatory to stricturing phenotype, and 1 UC patient progressed from left-sided to extensive. Among controls, 1 patient with CD progressed from inflammatory to penetrating phenotype. Two cases and 1 control discontinued biologics during follow-up. One case with ulcerative colitis, extensive colitis subtype, had been started on infliximab 2 years before bariatric surgery. In anticipation of the procedure, medication was held 2 months before Roux-en-Y. At 15-month follow-up, the patient continued to do well clinically, off of any IBD-specific medications, with a follow-up colonoscopy showing no evidence of active disease. At 4-year follow-up, the patient continued to do well off infliximab. Another case had been on a combination of anti–tumor necrosis factor (anti-TNF) and azathioprine that was stopped before surgery and not restarted after bariatric surgery. The patient developed an enterocutaneous fistula after surgery, which was thought to be a surgical complication in the absence of luminal active disease, and remained off of therapy. One control patient discontinued adalimumab due to a rash.
Safety Outcomes
The safety of bariatric surgery was evaluated across 31 IBD patients for whom the details of the performed surgical procedure and follow-up information were available. Five patients (16.1%) were readmitted within 90 days after surgery (Table 4); 3 of these cases were admitted for dehydration. Four patients required a re-operation. One patient had a complicated course worth noting. She was diagnosed with ileocolonic Crohn's disease 19 years prior and had multiple small bowel resections for penetrating complications. She had a history of perianal disease and at 1 point required an anti-TNF and thiopurine. However, at the time of surgery, she was in clinical remission on mesalamine only. The laparoscopic procedure had to be converted to open and was complicated by an anastomotic leak with subsequent development of an enterocutaneous fistula. She required total parenteral nutrition and unfortunately developed multiple episodes of sepsis, included septic arthritis. She eventually had the fistula closed surgically. She was lost to follow-up 9 years after surgery without having undergone endoscopic evaluation of her CD.
Table 4.
Surgical Complications After Bariatric Surgery
| Ulcerative Colitisa,b (n = 18) | Crohn's Diseasea,b (n = 13) | |
|---|---|---|
| Readmission within 90 d | 1 (5.6) | 4 (30.7) |
| Reoperation | 1 (5.6) | 3 (23.1) |
| Anastomotic leak | 2 (11.1) | 1 (7.7) |
| Reversal | 1 (5.6) | - |
| Incisional hernia | 1 (5.6) | 2 (15.4) |
| Small bowel obstruction | 2 (11.1) | - |
| Anastomotic ulcer | - | - |
| Stricture | 1 (5.6) | - |
aSurgical information was unavailable for 12 patients with UC and 5 patients with CD.
bDifferences were not statistically significant.
Literature Review
We identified 94 published cases of bariatric procedures being performed on patients with IBD (Table 5).15, 17, 19–23 Most patients had CD (64.9%). Complications were infrequent, and many patients had either stable or improved disease activity, with 6 (6.4%) having a flare of their disease and 3 (3.2%) requiring surgery for their IBD.
Table 5.
Literature on Bariatric Surgery in Patients With Inflammatory Bowel Disease
| Reference | No. Patients (Total/CD/UC) | IBD Medications | EWL, % | Complications | IBD Course |
|---|---|---|---|---|---|
| Colombo et al.15 | 6 (5/1) | ASA (4) Immunomodulator (3) Biologic (3) Steroids (6) |
74.5 ± 11.2 | Gastric suture bleeding in 1 patient | 3 patients had concurrent surgery for IBD; all patients were able to discontinue steroids by 1 year, though 1 had endoscopic disease |
| Aelfers et al.18 | 45 (29/16) | None (18) ASA (10) Immunomodulator (13) Biologic (3) Steroids (5) |
78.6 ± 29.3 | 1 bleeding anastomotic ulcer, 1 obstructive pyelonephritis, and pancreatitis |
3 flares, all >1 year after surgery |
| Aminian et al.17 | 20 (7/13) | None (9) | 58.9 ± 21.1 | 1 wound infection, 1 port-site hernia, 2 converted to open procedures, 1 biliary pancreatitis, 1 marginal ulcer, 5 dehydration, 1 PE | 7 patients were able to decrease IBD medications; 2 patients had IBD flares |
| Keidar et al.19 | 10 (8/2) | None (3) ASA (6) Biologic (1) |
71 | 1 staple line leakage requiring completion total gastrectomy with esophago-jejunostomy | 3 patients were able to stop ASA; 1 IBD flare treated with ASA |
| Lascano et al.20 | 1 (0/1) | ASA Immunomodulator Steroids |
80 | None | Symptomatic improvement but still endoscopic disease |
| Ungar et al.21 | 4 (4/0) | ASA (2) Immunomodulator (3) Biologic (1) |
60 | 1 staple line bleeding | All patients remained in remission |
| Honore et al.22 | 8 (8/0) | ASA (3) MTX (2) Immunomodulator (4) Biologic (4) |
56.5 | None | All patients remained in remission |
Abbreviations: ASA, aminosalicylate; EWL, excess weight loss; MTX, methotrexate.
DISCUSSION
In this study, we describe the largest case series of IBD patients who underwent bariatric surgery. We also conducted a case–control study evaluating the impact of weight loss after bariatric surgery on the long-term IBD disease course. We found that after bariatric surgery, there were numerically fewer IBD complications, specifically less rescue corticosteroid usage and IBD-related surgeries, compared with control IBD patients matched for age, sex, IBD phenotype, disease location, and disease duration, despite a higher BMI after bariatric surgery compared with IBD controls who had not undergone bariatric surgery. Bariatric surgery in this group of IBD patients was also associated with postoperative complications, though 60% of patients were on an either an immunomodulator or an anti-TNF or their combination.
In this study, patients who underwent bariatric surgery had a 12-kg/m2 decrease in BMI, which was comparable to the weight loss observed in a recently published meta-analysis of bariatric surgery in non-IBD patients.24 The rate of complications in that study was higher, but that study had a broader definition of complications.24 The rates of anastomotic leaks and re-operation were higher in our study than in a prospective study of bariatric surgery.25
In this study, we also found that patients with IBD who underwent bariatric surgery had numerically fewer overall IBD-related complications, including need for corticosteroids, compared with matched controls. This did not achieve statistical significance but may have been limited by small sample size. Overall, median follow-up was similar (~7 years) among both case and control IBD patients. Our findings are consistent with previous case series suggesting that bariatric surgery may improve inflammatory bowel disease activity.15, 19 In a series of 6 patients who were on corticosteroids at the time of bariatric surgery, all were able to discontinue corticosteroids by 1 year, although 1 patient had endoscopic disease activity.15 Notably, however, 3 of the patients had concurrent IBD-related surgery performed at the time of bariatric surgery.15 Aelfers et al. reported a series of 45 patients. Only 3 patients experienced an IBD flare during follow-up, and this was >1 year after bariatric surgery.19 Similarly, Aminian and colleagues17 reported a dose reduction in IBD medications in 7 patients and symptomatic improvement in 2 additional patients. Keidar and coworkers20 similarly reported that 3 patients were able to stop their 5-aminosalicylate medications after bariatric surgery.
The potential benefits of weight loss after bariatric surgery in IBD may be due to a decrease in the low chronic pro-inflammatory state (TNF-α -sink) associated with obesity, specifically reductions in high-sensitivity C-reactive protein, tumor necrosis factor-alpha, and interleukin-6, and consequently a lesser requirement for rescue therapy with corticosteroids.10, 26 Another potential mechanism for the beneficial effects of bariatric surgery in IBD patients may be mediated through changes in the microbiome.27, 28 Recent studies have highlighted the role of the microbiome in response to anti-integrins and mediating the efficacy of thiopurines.29, 30 Interestingly, studies have shown the changes in microbiome to occur after bariatric surgery independent of the numerical change in BMI.31 This is consistent with our observation that IBD patients who underwent bariatric surgery continued to have a higher BMI despite the weight loss, compared with matched IBD controls who had not undergone bariatric surgery. Additional benefits of weight loss after bariatric surgery may extend to reducing the technical challenges of constructing a stoma and reduction in the foreshortening of mesentery in obese patients in ileal pouch–anal anastomosis construction.
Although several other studies have suggested that bariatric surgery can be performed on IBD patients without inducing a flare, the main strength of our study is the attempt to understand the impact of weight loss after bariatric surgery on IBD course and activity by including a matched control group to compare IBD disease course after surgery. The 2 groups have been matched for variables known to influence the disease course of IBD, including sex, type of IBD, IBD diagnosis date, disease duration at the start of the follow-up period, disease location, and phenotype of disease, along with similar duration of follow-up across both cases and controls. In addition, we describe the largest case series from 2 tertiary referral centers for both IBD and bariatric surgery.
The number of postoperative complications was higher in our study than in some other studies.14, 19, 21, 23 The rate of anastomotic leaks was lower in a recent study by our group using an administrative data set.14 However, this study could not account for what medications patients were on. The frequency of complications in our study appears similar to smaller case series (Table 5).15, 17, 20, 22 Recent studies have suggested that being on an anti-TNF does not increase infectious postoperative complications.32, 33 However, our study was too small to assess whether there was a difference in surgical complications or IBD flare depending on the timing of biologic exposure. The rate of reoperation in a recent meta-analysis of bariatric surgery on non-IBD patients was 6%–7%.24 The reoperation rate was similar in UC patients in our study, but CD patients had a much higher reoperation rate.
Our study has several limitations. It was retrospective in design, making it prone to selection bias. Though it is the largest case series of IBD patients who underwent bariatric surgery, the numbers are still relatively modest. Our numbers were too small to compare the difference in IBD outcomes between the different bariatric procedures. We had to exclude several cases due to inability to find matched controls. As the study was based at 2 referral centers for both IBD and bariatric surgery, our results may not be generalizable to other settings. However, many of the patients had their bariatric procedure performed at another institution while receiving IBD care at our institutions. In addition, not all patients had objective information about disease activity at the time of surgery, such as C-reactive protein, fecal calprotectin, imaging, or endoscopy. Not surprisingly, most patients had mild or inactive disease when they underwent bariatric surgery. Our results are not generalizable to patients with severe, active disease. No patients in our study were on newer medications that have been approved for IBD in recent years. The risk of complications may be different with newer medications that have been approved for IBD in recent years. No patients in our study were on any of these medications. It is possible that changes in surgical technique and patient selection over time may have affected our results.
In summary, we have shown that bariatric surgery and resultant weight loss in IBD patients are associated with a reduced number of IBD complications after bariatric surgery compared with matched IBD controls who have not undergone a bariatric procedure. The frequency of complications in our study was higher than reported in the published literature and needs to be considered in deciding whether to pursue bariatric surgery in a patient with IBD. Further research is needed to confirm our findings in a prospective study design and elucidate the mechanisms that may underlie the improvement in IBD disease activity after bariatric surgery and whether these extend to the weight loss achieved through endoscopic bariatric procedures in the carefully selected IBD patient.
Supported by: P.D. is supported by a Junior Faculty Development Award from the American College of Gastroenterology. M.A.C. is supported by DK109384, a Crohn's and Colitis Foundation Daniel H. Present Senior Research Award (Ref. 370763), and philanthropic support from the Givin' it all for Guts Foundation (https://givinitallforguts.org) and the Lawrence C. Pakula MD IBD Research Innovation and Education Fund. The work performed in this paper was additionally supported by grants provided by the National Institutes of Health through the Washington University in Saint Louis' Digestive Disease Research Core (P30 DK052574).
Conflicts of interest: Manuel B. Braga Neto, Martin H. Gregory, Guilherme P. Ramos, David H. Bruining, Fateh Bazerbachi, Barham K. Abu Dayyeh, Vladimir M. Kushnir, and Laura E. Raffals have no relevant conflicts of interest to disclose.
Edward V. Loftus Jr.: consulting for AbbVie, Allergan, Amgen, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Janssen, Pfizer, Takeda, UCB; research support from AbbVie, Amgen, Celgene, Genentech, Gilead, Janssen, Medimmune, Pfizer, Robarts Clinical Trials, Seres, Takeda, UCB. Matthew A. Ciorba: speakers' bureau or consulting for AbbVie, Pfizer, Takeda, UCB; grants from Incyte, no relation to this work. Parakkal Deepak: speakers' bureau for Abbvie; advisory board for Pfizer and Janssen; research grant from Takeda.
REFERENCES
- 1. Harpsøe MC, Basit S, Andersson M, et al. . Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43:843–855. [DOI] [PubMed] [Google Scholar]
- 2. Hales CM, Carroll MD, Fryar CD, et al. . Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;(288):1–8. [PubMed] [Google Scholar]
- 3. NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. English WJ, DeMaria EJ, Brethauer SA, et al. . American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14:259–263. [DOI] [PubMed] [Google Scholar]
- 5. Mamula P, Telega GW, Markowitz JE, et al. . Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–2010. [DOI] [PubMed] [Google Scholar]
- 6. Nic Suibhne T, Raftery TC, McMahon O, et al. . High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–e248. [DOI] [PubMed] [Google Scholar]
- 7. Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. . Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2857–2863. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen NH, Ohno-Machado L, Sandborn WJ, et al. . Obesity is independently associated with higher annual burden and costs of hospitalization in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:709–718.e7. [DOI] [PubMed] [Google Scholar]
- 9. Singla MB, Eickhoff C, Betteridge J. Extraintestinal manifestations are common in obese patients with Crohn's disease. Inflamm Bowel Dis. 2017;23:1637–1642. [DOI] [PubMed] [Google Scholar]
- 10. Singh S, Dulai PS, Zarrinpar A, et al. . Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stidham RW, Waljee AK, Day NM, et al. . Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in Crohn's disease. Inflamm Bowel Dis. 2015;21:1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiran RP, Remzi FH, Fazio VW, et al. . Complications and functional results after ileoanal pouch formation in obese patients. J Gastrointest Surg. 2008;12:668–674. [DOI] [PubMed] [Google Scholar]
- 13. Duchesne JC, Wang YZ, Weintraub SL, et al. . Stoma complications: a multivariate analysis. Am Surg. 2002;68:961–966; discussion 966. [PubMed] [Google Scholar]
- 14. Bazerbachi F, Sawas T, Vargas EJ, et al. . Bariatric surgery is acceptably safe in obese inflammatory bowel disease patients: analysis of the nationwide inpatient sample. Obes Surg. 2018;28:1007–1014. [DOI] [PubMed] [Google Scholar]
- 15. Colombo F, Rizzi A, Ferrari C, et al. . Bariatric surgery in patients with inflammatory bowel disease: an accessible path? Report of a case series and review of the literature. J Crohns Colitis. 2015;9:185–190. [DOI] [PubMed] [Google Scholar]
- 16. Cañete F, Mañosa M, Clos A, et al. . Review article: the relationship between obesity, bariatric surgery, and inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:807–816. [DOI] [PubMed] [Google Scholar]
- 17. Aminian A, Andalib A, Ver MR, et al. . Outcomes of bariatric surgery in patients with inflammatory bowel disease. Obes Surg. 2016;26:1186–1190. [DOI] [PubMed] [Google Scholar]
- 18. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 19. Aelfers S, Janssen IMC, Aarts EO, et al. . Inflammatory bowel disease is not a contraindication for bariatric surgery. Obes Surg. 2018;28:1681–1687. [DOI] [PubMed] [Google Scholar]
- 20. Keidar A, Hazan D, Sadot E, et al. . The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis. 2015;11:132–136. [DOI] [PubMed] [Google Scholar]
- 21. Lascano CA, Soto F, Carrodeguas L, et al. . Management of ulcerative colitis in the morbidly obese patient: is bariatric surgery indicated? Obes Surg. 2006;16:783–786. [DOI] [PubMed] [Google Scholar]
- 22. Ungar B, Kopylov U, Goitein D, et al. . Severe and morbid obesity in Crohn's disease patients: prevalence and disease associations. Digestion. 2013;88:26–32. [DOI] [PubMed] [Google Scholar]
- 23. Honoré M, McLeod G, Hopkins G. Outcomes of laparoscopic sleeve gastrectomy in Crohn's disease patients: an initial Australian experience. ANZ J Surg. 2018;88:E708–E712. [DOI] [PubMed] [Google Scholar]
- 24. Chang SH, Stoll CR, Song J, et al. . The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sjöström L, Lindroos AK, Peltonen M, et al. ; Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 26. Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, et al. . Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–955. [DOI] [PubMed] [Google Scholar]
- 27. Liu R, Hong J, Xu X, et al. . Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–868. [DOI] [PubMed] [Google Scholar]
- 28. Magouliotis DE, Tasiopoulou VS, Sioka E, et al. . Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27:1345–1357. [DOI] [PubMed] [Google Scholar]
- 29. Ananthakrishnan AN, Luo C, Yajnik V, et al. . Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017;21:603–610.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oancea I, Movva R, Das I, et al. . Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut. 2017;66:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tremaroli V, Karlsson F, Werling M, et al. . Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fumery M, Seksik P, Auzolle C, et al. ; REMIND Study Group Investigators Postoperative complications after ileocecal resection in Crohn's disease: a prospective study from the REMIND Group. Am J Gastroenterol. 2017;112:337–345. [DOI] [PubMed] [Google Scholar]
- 33. Gregory MH, McKinnon A, Stwalley D, et al. . Anti-tumour necrosis factor therapy for inflammatory bowel diseases do not impact serious infections after arthroplasty. J Crohns Colitis. 2019;13:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]