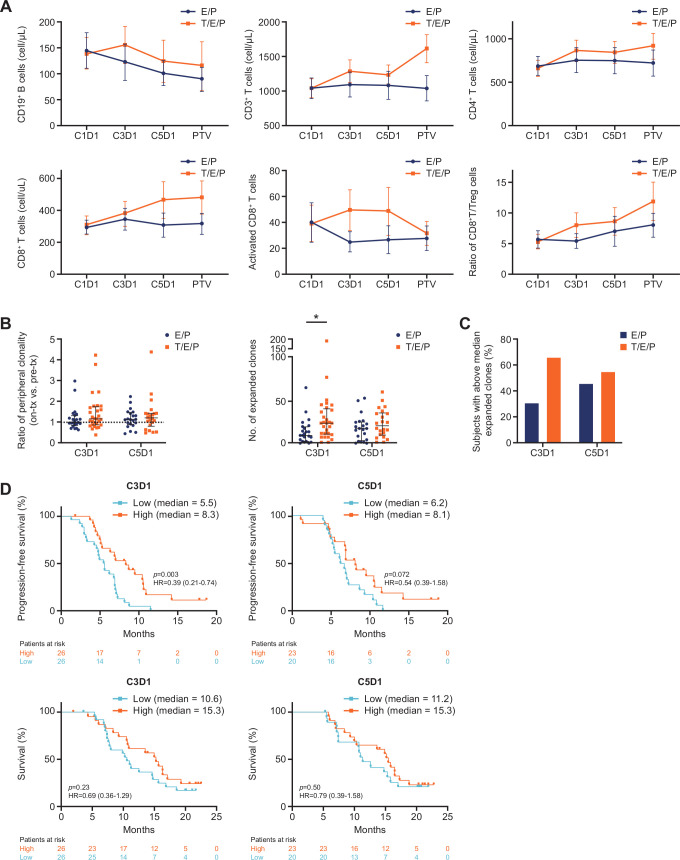
Figure 5.
Addition of trilaciclib to E/P preserved and enhanced lymphocyte counts and function in patients with SCLC. (A) Mean absolute cell count (cells/µL) for B-cell and T-cell subsets and activated CD8+ T cells (CD3+CD8+CD38+HLA-DR+), and ratio of absolute whole blood cell counts for CD8+ T cells to Tregs (CD3+CD4+CD25+CD127low), at the indicated time points. Error bars represent 95% CIs. (B) Per-patient T-cell repertoire analysis comparing mean ratio of clonality versus pretreatment level (C1D1) and number of expanded T-cell clones at the indicated time points. Bars represent median and IQR. (C) Proportion of patients with high (≥median) number of expanded T-cell clones at C3D1 (≥15 clones) and C5D1 (≥17 clones). (D) Progression-free and overall survival analysis of all patients with low (<median) and high (≥median) numbers of expanded T-cell clones on C3D1 and C5D1. *P=0.0098. C1D1, cycle 1 day 1; C3D1, cycle 3 day 1; C5D1, cycle 5 day 1; D, day; E/P, etoposide and carboplatin; T/E/P, trilaciclib prior to etoposide and carboplatin; PTV, post-treatment visit; SCLC, small cell lung cancer; Treg, T-regulatory cell.