Figure 3.
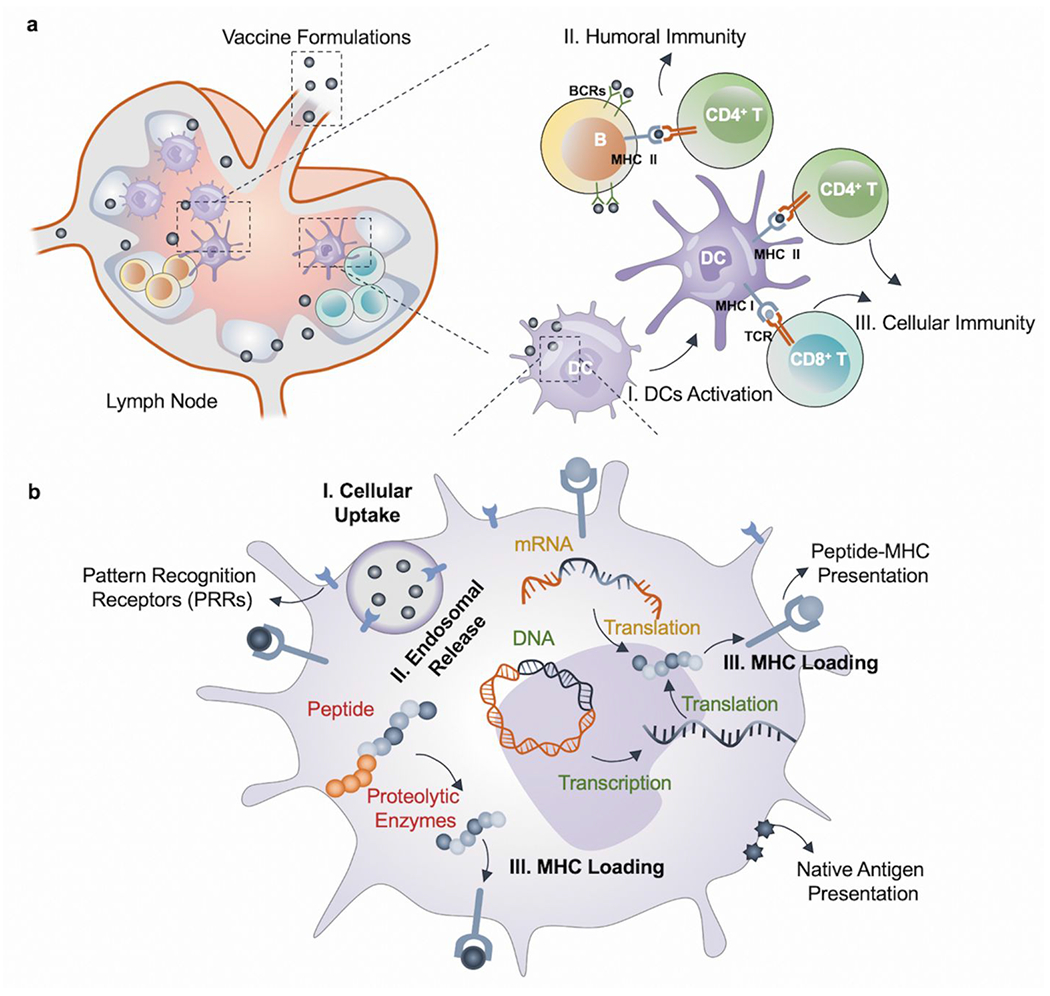
Action mechanisms of vaccine formulations. (a) Vaccines generate humoral and cellular immunity within lymph nodes (LNs): I. DCs can process the antigens and present the peptide fragments via both MHC class I and class II molecules. II. B cells can directly recognize the antigens via BCRs and present the antigenic peptide fragments by MHC class II to helper T cells (CD 4+). Stimulated B cells can subsequently initiate a humoral immune response. III. Cytotoxic T cells (CD 8+) can recognize the antigenic peptide fragments presented by MHC class I through TCRs and trigger the cellular immune response.12,45 (b) Intracellular response of DCs to antigen presentation for different types of vaccines through PRRs. I. Vaccine formulations can be effectively uptaken by cells, followed by II. Endosomal release. Before III. MHC loading of antigen peptide, peptide vaccine undergoes enzymatic processing, DNA vaccine undergoes transcription and translation, and mRNA vaccine undergoes translation.24,27,37
