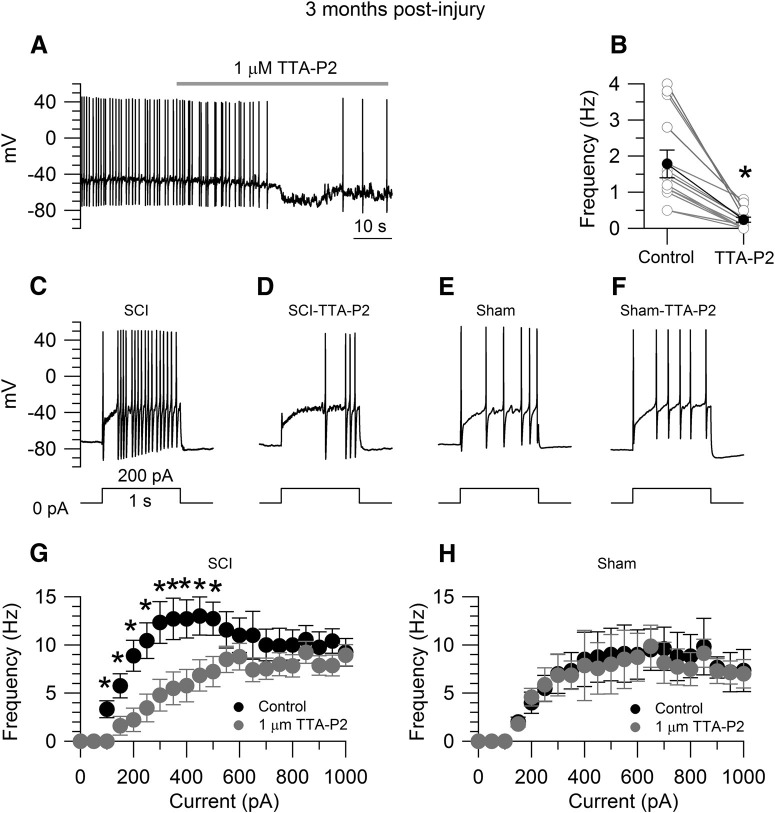
Figure 6.
Effects of TTA-P2 on the increased excitability of SCI-nociceptors. A, Spontaneous activity in a SCI-nociceptor isolated at three months postinjury. The firing frequency was reduced from 1.4 Hz (in control) to 0.2 Hz in the presence of 1 μm TTA-P2. B, In collected results, the firing frequency was reduced from 1.8 ± 0.3 Hz (in control) to 0.3 ± 0.1 Hz in the presence of 1 μm TTA-P2 (n = 13). Paired t test, *p < 0.05. Current-clamp recordings in a SCI-nociceptor isolated at three months postinjury showing the evoked firing in response to 200 pA of current injection in control (C) and in the presence of 1 μm TTA-P2 (D). Current-clamp recordings in a sham-nociceptor isolated at three months postlaminectomy showing the evoked firing in response to 200 pA of current injection in control (E) and in the presence of 1 μm TTA-P2 (F). G, f-I relationship in response to current injections from 0 to 1000 pA (50-pA increment) in SCI-nociceptors in control (black dots) and in the presence of 1 μm TTA-P2 (gray dots, n = 19). H, f-I relationship in response to current injections in sham-nociceptors in control (black dots) and in the presence of 1 μm TTA-P2 (gray dots, n = 15). The f-I curves were generated from the same neurons before and after TTA-P2 application. The f-I curve was first generated in control. Then, the same cell was held at the resting potential and continuously perfused with 1 μm TTA-P2 for 3 min before a second f-I curve was generated in the presence of 1 μm TTA-P2. G, H, Two-way ANOVA followed by Sidak's post hoc comparison, *p < 0.05. Data are reported as mean ± SEM.