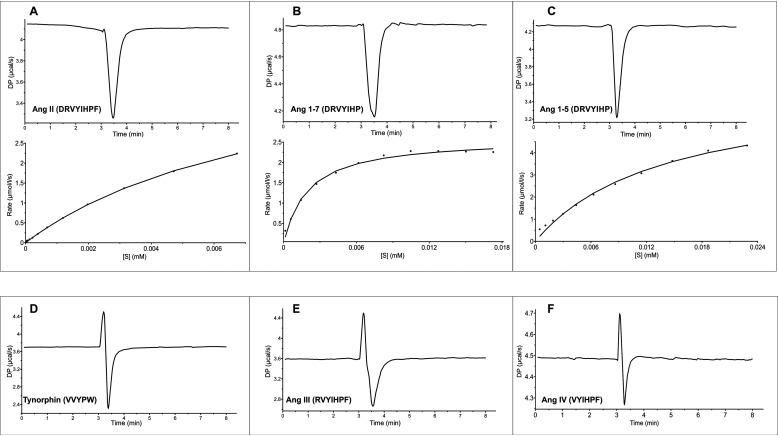
Figure 9.
RAS peptides can be “good” or “bad” substrates of DPP3. RAS peptides turned over by DPP3 (DP), thus acting as “good” substrates (A–C). Raw data showing the heat change of the reaction as a function of time (top panels) and fitted curve for the rate of reaction (bottom panels) of Ang II (A), Ang(1–7) (B), and Ang(1–5) (C). Some RAS peptides demonstrated both endothermic and exothermic behavior, thus acting as “slow” substrates of DPP3 (D–F). The biphasic peaks were most likely due to binding to DPP3 and a subsequent slow turnover event. Raw data showing the heat change of the reaction in the case of slow substrates, tynorphin (D), Ang III (E), and Ang IV (F). Curve fitting was not possible in the case of slow substrates. The reaction was started by injecting 5 µl of 2 mm angiotensin peptides to the calorimetric cell containing 20 μm hDPP3. The data represent three or more technical replicates from two biological replicates.