Figure 1. Flow of Patients in the Study of Tranexamic Acid During Air and Ground Prehospital Transport (STAAMP) Trial.
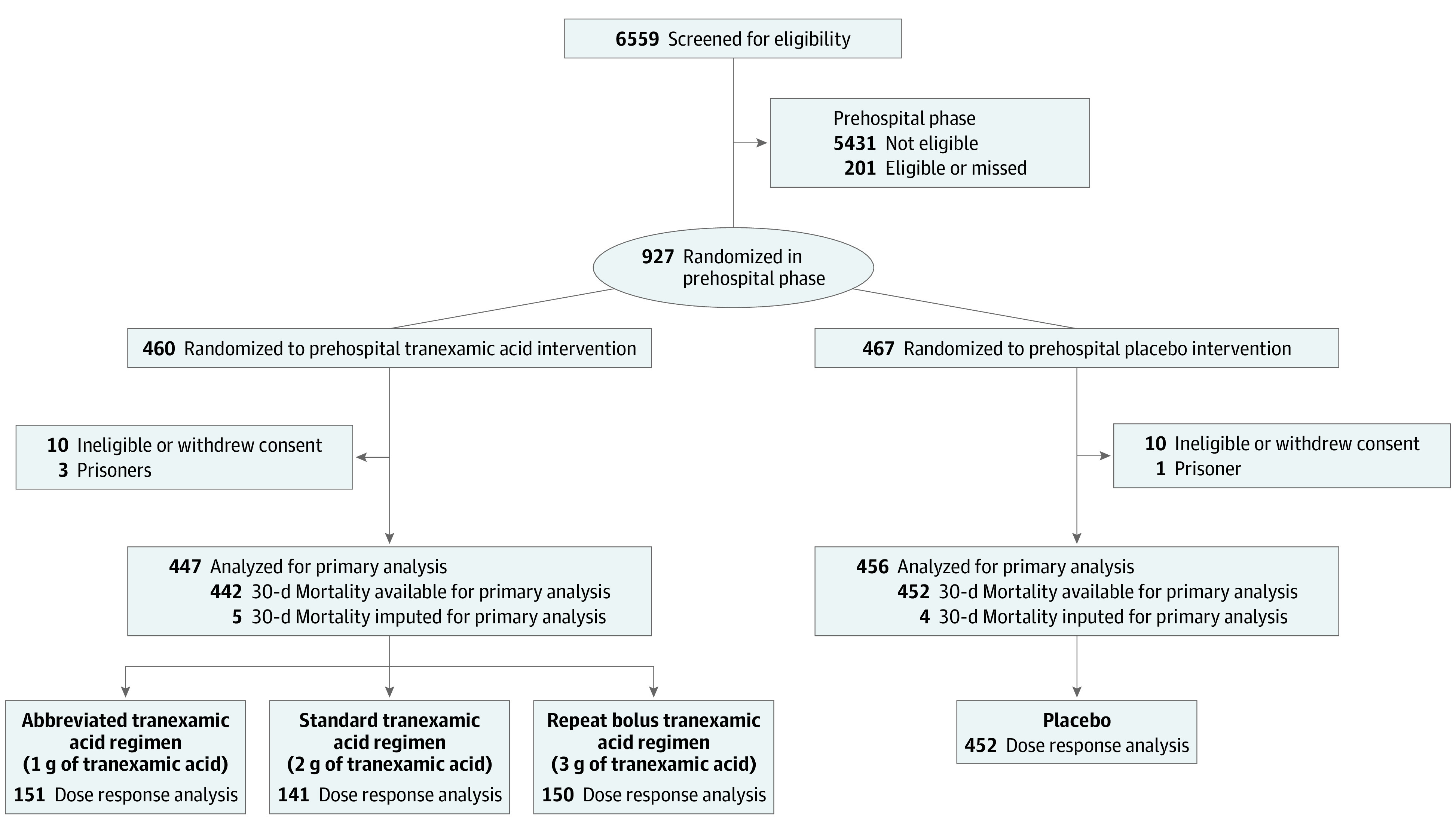
Screening, randomization, and follow-up of the study participants. Per site institutional review board requirements, data from participants who withdrew consent or were excluded based on ineligibility in certain situations could not be included for the intention-to-treat analysis. Multiple imputation was performed for the 9 participants missing the primary outcome for the primary analysis as prespecified in the study protocol. Dose response analyses were performed on those with 30-day mortality data available.
