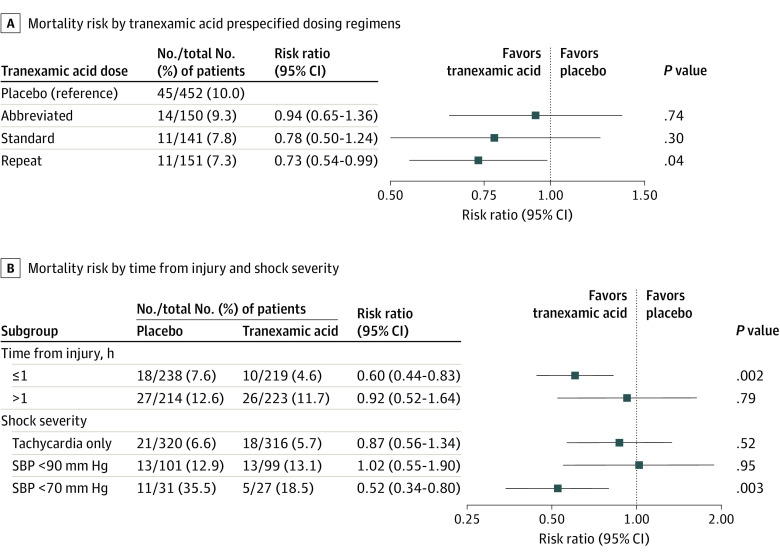
Figure 3. Prespecified Tranexamic Acid Dose Response Analysis, Time to Intervention, and Shock Severity Post Hoc Subgroup Analysis for 30-Day Mortality.
A, Risk of 30-day mortality across tranexamic acid prespecified dosing regimens, accounting for site clustering. All risk ratios are in reference to the placebo group. The abbreviated dose represents a single 1-g bolus dose. The standard dose represents a 2-g dose administered as a 1-g bolus dose followed by a 1-g infusion during 8 hours. The repeat dose represents a 3-g dose administered as 2 separate 1-g boluses followed by a 1-g infusion during 8 hours. The repeat dose had lower risk of 30-day mortality than placebo group. B, Risk of 30-day mortality of the tranexamic acid group compared with placebo accounting for site clustering across post hoc subgroups for time of tranexamic acid administration from injury and shock severity based on qualifying inclusion vital signs. The dotted vertical line represents a risk ratio of 1.0 (no difference between groups). The squares represent the point estimate of the risk ratio, with the horizontal solid lines representing the 95% CIs. Time of tranexamic acid administration from injury was stratified by 1 hour or less and greater than 1 hour. The risk of 30-day mortality was lower in the tranexamic acid group when the drug was administered within 1 hour of injury. The risk of 30-day mortality was lower in the tranexamic acid group among patients in severe shock with systolic blood pressure less than 70 mm Hg based on qualifying inclusion vital signs. SBP indicates systolic blood pressure.