Abstract
Background
Rate of SARS‐CoV‐2 infection and impact of liver fibrosis stage upon infection rates in persons with hepatitis C virus (HCV) infection are unknown.
Methods
We retrospectively analysed the Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES), a well‐established database of HCV‐infected Veterans in care. We excluded those with missing FIB‐4 score and those with HIV or hepatitis B virus co‐infection. We determined the number of persons tested, proportion who tested positive for SARS‐CoV‐2 and the infection rate by age and liver fibrosis stage.
Results
Among 172,235 persons with HCV, 14,305 (8.3%) were tested for SARS‐CoV‐2 infection and 892 (6.2%) tested positive. Those with SARS‐CoV‐2 infection were older, more likely to be Black (55.2% vs 37.8%), obese (body mass index >30 kg/m2 36.2% vs 29.7%) and have diabetes or stroke (P < .0001 for all comparisons). Mean FIB‐4 scores and proportion of persons with cirrhosis (based on a FIB‐4 > 3.25) were similar in both groups. Incidence rate/1,000 tested persons was much higher among Blacks (88.4; 95% CI 81.1, 96.2) vs Whites (37.5; 95% CI 33.1, 42.4) but similar among those with cirrhosis (FIB‐4 > 3.25). The rates were also similar among those who were untreated for HCV vs those treated with or without attaining a sustained virologic response.
Conclusions
Testing rates among persons with HCV are very low. Persons with infection are more likely to be Black, have a higher body mass index and diabetes or stroke. The degree of liver fibrosis does not appear to have an impact on infection rate.
Keywords: ERCHIVES, SARS‐CoV‐2, hepatitis C virus, liver fibrosis
Key points.
Testing rates among persons with HCV are very low.
Persons with HCV who have COVID‐19 infection are more likely to be Black, have a higher body mass index and diabetes or stroke.
The degree of liver fibrosis does not appear to have an impact on infection rate.
1. INTRODUCTION
As of 9 August 2020, >20 million persons have been infected and over 730 000 have died of SARS‐CoV‐2 infection worldwide. While infection rates appear to have diminished significantly in some countries, the pandemic rages on in others, particularly in the Americas. While primarily a respiratory illness, SARS‐CoV‐2 may affect multiple organ systems. Effects on cardiovascular, 1 renal, 2 gastrointestinal, 3 hepatic, 3 , 4 endocrine 5 and neurologic systems have been reported. Gastrointestinal symptoms may be present in up to 15% of patients and abnormal liver enzymes in up to 36% of the hospitalized patients. 6 , 7 The incidence of SARS‐CoV‐2 infection among persons with hepatitis C virus (HCV) infection, and its association with degree of liver fibrosis are unknown. Whether persons with HCV experience more severe disease or higher mortality is also unknown. We undertook this study to determine the infection rate and clinical characteristics of SARS‐CoV‐2 infection among persons with HCV infection.
2. MATERIALS AND METHODS
We used the Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES) for the current study. Creation of ERCHIVES has been described in numerous previous publications. 8 , 9 , 10 , 11 , 12 , 13 Briefly, all Veterans with a positive HCV antibody test from 2001 onwards are identified through the VA Corporate Data Warehouse (CDW). Age‐, sex‐ and race‐matched controls are identified based on a negative HCV antibody test in the same year. Clinical, laboratory, pharmacy, anthropometric and vital signs data are retrieved for each case and control using established definitions and algorithms. Smoking status is retrieved from the Health Factors dataset. Mortality data are cross‐validated and cause of death data are retrieved from the Centers for Disease Control and Prevention's National Death Index. 13 Data are updated annually to include Veterans with newly diagnosed HCV and corresponding controls.
For the current study, we identified all Veterans in the ERCHIVES database with a positive HCV antibody as well as a positive HCV RNA. We excluded those with missing laboratory values to calculate the FIB‐4 score as the marker for liver fibrosis stage. We also excluded those with HIV or hepatitis B virus co‐infection. Comorbidities were defined using established and published definitions. 8 , 9 , 10 , 11 , 12 , 13 The diagnosis of SARS‐CoV‐2 infection was confirmed from the VA CDW, where a standard nasopharyngeal swab is tested using RT‐PCR to confirm the diagnosis. Liver fibrosis stage was calculated using the FIB‐4 score using an average of two values closest to but before baseline.
We compared the baseline characteristics of all Veterans with HCV in the ERCHIVES cohort who were diagnosed with SARS‐CoV‐2 with those who tested negative for SARS‐CoV‐2. We calculated incidence rates per 1000 persons with 95% confidence intervals by age group, sex, race, liver fibrosis stage and HCV treatment status.
We used SAS® (version 9.4, SAS Institute Inc.) for analyses.
The study was approved by the Institutional Review Board at VA Pittsburgh Healthcare System, Pittsburgh, PA.
3. RESULTS
Among 781 271 persons in the ERCHIVES database, 190 771 had a positive HCV antibody and at least one positive HCV RNA. After excluding 13 529 with missing laboratory values to calculate the FIB‐4 score, 3227 with HIV co‐infection and 1780 with HBV co‐infection, there were 172 235 persons in the final evaluable sample (Figure 1). Among these, 14 305 (8.3%) Veterans were tested for SARS‐CoV‐2 infection. Among those tested, 892 (6.2%) tested positive. Baseline characteristics of persons with and without SARS‐CoV‐2 infection compared are provided in Table 1. Compared with those without SARS‐CoV‐2 infection, those with SARS‐CoV‐2 were older (median age 61 vs 60 years), more likely to be Black (55.2% vs 37.8%), obese (% with body mass index >30 kg/m2 36.2% vs 29.7%) and more likely to have a diagnosis of diabetes and stroke (P < .0001 for all comparisons). Mean FIB‐4 scores and proportion of persons with advanced fibrosis or cirrhosis (based on a FIB‐4 > 3.25) were similar in both groups. Proportion of persons treated for HCV (70.4% vs 71.3%; P = .5) and proportion who had attained sustained virologic response among those treated (91.7% vs 92.0%; P = .8) were also similar among those with SARS‐CoV‐2 infection compared with those without infection (Table 1).
FIGURE 1.
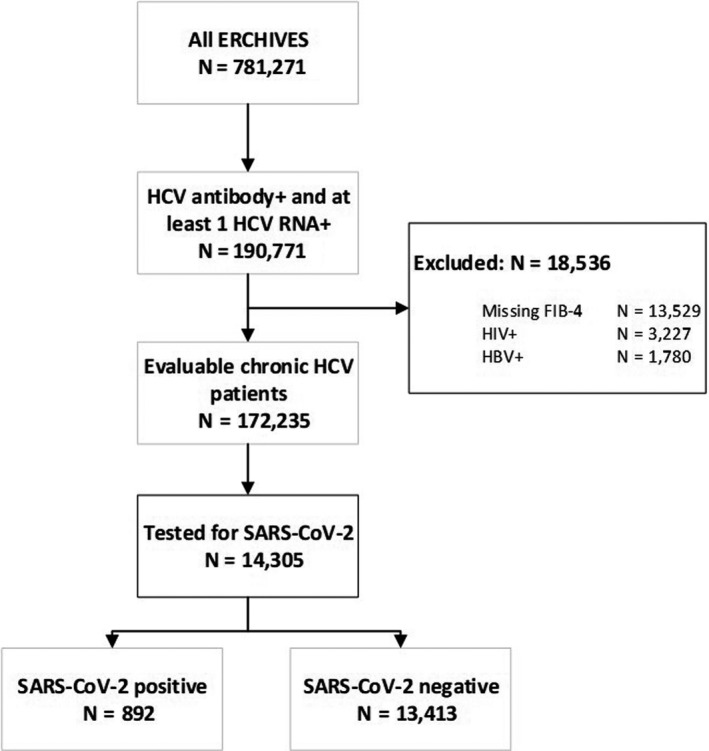
Study flow sheet and cohort construction
TABLE 1.
Baseline characteristics of HCV+ persons with and without SARS‐CoV‐2 infection
| SARS‐CoV‐2 POS | SARS‐CoV‐2 NEG | P‐value | |
|---|---|---|---|
| N = 892 | N = 13,413 | ||
| Median age, y (IQR) | 61 (56, 65) | 60 (55, 64) | <.0001 |
| Race, % | <.0001 | ||
| White | 28.14% | 47.98% | |
| Black | 55.16% | 37.81% | |
| Hispanic | 5.27% | 4.03% | |
| Other/unknown | 11.43% | 10.18% | |
| Sex, % male | 95.96% | 95.74% | .74 |
| Body mass index | |||
| Mean, SD | 28.4 (5.8) | 27.9 (5.8) | .02 |
| >30 | 36.22% | 29.69% | <.0001 |
| Median HCV RNA, log10 (IQR) | 5.8 (1.3, 7.1) | 5.8 (1.2, 7.3) | .35 |
| FIB‐4 score | |||
| Mean FIB‐4 score (SD) | 2.4 (2.6) | 2.5 (3.2) | .84 |
| FIB‐4 > 3.25, % | 15.25% | 17.33% | .11 |
| Comorbidities, % | |||
| Hypertension | 66.37% | 63.25% | .06 |
| Diabetes | 35.09% | 28.41% | <.0001 |
| Coronary artery disease | 0.45% | 0.57% | .63 |
| Stroke | 7.29% | 4.20% | <.0001 |
| Cancer | 10.65% | 10.86% | .84 |
| Chronic obstructive pulmonary disease | 14.80% | 14.53% | .83 |
| Smoking, % | <.0001 | ||
| Current | 46.41% | 59.26% | |
| Former | 27.91% | 21.32% | |
| Never | 21.52% | 15.78% | |
| Unknown | 4.15% | 3.64% | |
| Alcohol use disorder, % | 22.87% | 28.20% | .001 |
| Drug use disorder, % | 28.92% | 35.47% | <.0001 |
| Treated for HCV, % | 70.40% | 71.33% | .55 |
| Attained sustained virologic response, % | 91.73% | 92.02% | .80 |
| Admitted to hospital | 35.87% | 30.49% | .001 |
| Admitted to ICU | 12.33% | 6.93% | <.0001 |
| Died | 6.17% | 1.71% | <.0001 |
The overall incidence rate per 1000 HCV‐infected persons (95% CI) was 62.4 (58.4, 66.4) (Table 2). Incidence rate was much higher among Blacks (88.4; 95% 81.1, 96.2) compared with Whites (37.5; 95% CI 33.1, 42.4) with no overlap in 95% confidence intervals. The incidence rates per 1000 HCV‐infected persons increased with increasing age with rate of 24.0 among those 18‐50 years old, 52.7 among >50‐60 years old, 60.7 among >60‐70 years old and 80.1 among >70 year old age groups. The incidence rate per 1000 HCV‐infected persons was 57.6 (95% CI 51.5, 64.2) among those with FIB‐4 < 1.45 (minimal or no fibrosis), 68.9 (95% CI 62.9, 75.4) among those with FIB‐4 of 1.45‐3.25 and 55.3 (95% CI 46.6, 65.1) among those with FIB‐4 > 3.25 (significant fibrosis or cirrhosis). The rates were similar among those who were untreated for HCV compared with those treated and attained sustained virologic response and those treated and did not attain a sustained virologic response (Table 2).
TABLE 2.
Incidence rates per 1,000 persons of COVID‐19 infection in various subgroups
| HCV+ (N = 14,305) | ||
|---|---|---|
| N | Rate (95% CI) | |
| Overall | 892 | 62.4 (58.4, 66.4) |
| Males | 856 | 62.5 (58.5, 66.7) |
| Females | 36 | 59.2 (41.8, 81) |
| Race | ||
| White | 251 | 37.5 (33.1, 42.4) |
| Black | 492 | 88.4 (81.1, 96.2) |
| Hispanic | 47 | 79.9 (59.3, 104.9) |
| Other/unknown | 102 | 69.5 (57, 83.8) |
| Age group | ||
| 18‐50 | 17 | 24 (14, 38.1) |
| >50‐60 | 95 | 52.7 (42.8, 64) |
| >60‐70 | 514 | 60.7 (55.7, 66) |
| >70 | 266 | 80.1 (71.1, 89.8) |
| HCV treatment | ||
| Untreated | 264 | 64.2 (56.9, 72.2) |
| Treated with SVR | 521 | 61.4 (56.4, 66.7) |
| Treated without SVR | 47 | 63.7 (47.2, 83.8) |
| Treated with SVR missing | 60 | 61.9 (47.5, 78.9) |
| Liver fibrosis stage | ||
| FIB‐4 < 1.45 | 308 | 57.6 (51.5, 64.2) |
| FIB‐4 1.45‐3.25 | 448 | 68.9 (62.9, 75.4) |
| FIB‐4 > 3.25 | 136 | 55.3 (46.6, 65.1) |
| Mortality | ||
| Overall | 55 | 3.8 (2.9, 5) |
| Among hospitalized | 32 | 2.2 (1.5, 3.2) |
| Among ICU | 19 | 1.3 (0.8, 2.1) |
We compared the incidence rate of SARS‐CoV‐2 infection in our HCV‐positive group with persons without HCV infection. The latter group consisted of all persons in ERCHIVES with a negative HCV antibody test and a negative HCV RNA (if done). Similar to the group with HCV, we excluded those with HIV and HBV co‐infection and retained those with laboratory data to calculate FIB‐4 score at baseline. Among 347 117 persons without HCV infection, the overall SARS‐CoV‐2 incidence rate per 1000 persons was 3.7 (95% CI 3.5, 3.9; P = 0.8 compared with HCV+ group). All‐cause mortality rate during the follow‐up period was 0.3/1000 persons for both groups (P = 0.6).
4. DISCUSSION
To our knowledge, this is the first study describing the testing rate and the incidence of SARS‐CoV‐2 infection in a large national population of persons with HCV infection. We also describe factors associated with infection.
We found that the persons with SARS‐CoV‐2 infection were more likely to be Black, have a higher body mass index and have diabetes or stroke. Higher rates of infection and poorer clinical outcomes have been described in racial/ethnic minorities and those with comorbidities. More recently, obesity has emerged as an independent risk factor for more severe disease. These associations have not been previously reported in persons with HCV infection. While our current study did not determine the impact of these factors upon mortality or severity of illness, the higher rate of infection coupled with existing literature of their impact upon outcomes should prompt an aggressive testing and monitoring strategy in these persons.
Several small studies (50‐363 patients) have assessed the impact of liver disease and/or cirrhosis upon severity of SARS‐CoV‐2 infection and overall mortality. 14 , 15 , 16 , 17 , 18 Presence of advanced fibrosis as measured by the FIB‐4 score, clinical liver disease or cirrhosis are all associated with a higher risk of more severe disease or higher mortality. The cause of liver disease, and more specifically the presence of HCV infection were not uniformly reported. Furthermore, there are no estimates of testing for SARS‐CoV‐2 and infection rates among persons with HCV and the impact of liver fibrosis or cirrhosis upon the testing or infection rates. We found that the degree of liver fibrosis as measured by the non‐invasive FIB‐4 score was identical and a similar proportion had advanced fibrosis or cirrhosis. Incidence rate was also similar regardless of the liver fibrosis stage at baseline. Additionally, treatment of HCV and attainment of sustained virologic response did not seem to affect the rate of infection.
Strengths of our study include a large national sample with relatively easy and free access to testing. However, lack of widespread testing limits the generalizability of our findings. Some Veterans may have been tested outside the VA healthcare system which may have skewed the results. Effect of various factors upon severity of disease and mortality requires further study.
5. CONCLUSIONS
This study provides compelling data regarding SARS‐CoV‐2 infection in persons with HCV infection with several notable points for clinicians and policymakers. The overall testing rate in persons with HCV is extremely low. When tested, infection appears to disproportionately affect minorities, obese persons and those with certain comorbidities. However, degree of liver fibrosis does not appear to increase the risk of infection. Similarly, infection rates are comparable among those who never received HCV treatment and those who received treatment with or without the attainment of viral eradication.
CONFLICT OF INTEREST
All authors have no potential conflict of interest to disclose.
AUTHOR CONTRIBUTION
AAB: Study design, data acquisition, data interpretation and writing of the manuscript; PY: Data acquisition and data analysis.
AUTHORSHIP STATEMENT
Dr Butt had complete access to data at all times and accepts the responsibility of the integrity of this article.
ACKNOWLEDGEMENTS
This material is the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System and the central data repositories maintained by the VA Information Resource Center, including the National Patient Care Database, Decisions Support System Database and Pharmacy Benefits Management Database. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Butt AA, Yan P. Rates and characteristics of SARS‐CoV‐2 infection in persons with hepatitis C virus infection. Liver Int. 2021;41:76–80. 10.1111/liv.14681
Handling Editor: Alessio Aghemo
REFERENCES
- 1. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA cardiology. 2020. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kunutsor SK, Laukkanen JA. Renal complications in COVID‐19: a systematic review and meta‐analysis. Ann Med. 2020;1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rokkas T. Gastrointestinal involvement in COVID‐19: a systematic review and meta‐analysis. Ann Gastroenterol. 2020;33:355‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarifian A, Zamiri Bidary M, Arekhi S, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2020. 10.1002/jmv.26314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubino F, Amiel SA, Zimmet P, et al. New‐onset diabetes in COVID‐19. N Engl J Med. 2020;383(8):789‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Cui M, Yang T, Yao P. Correlation between gastrointestinal symptoms and disease severity in patients with COVID‐19: a systematic review and meta‐analysis. BMJ Open Gastroenterol. 2020;7(1):e000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y, Li H, Guo X, et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID‐19 patients: a systematic review and meta‐analysis. Hepatol Int. 2020. 10.1007/s12072-020-10074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose‐dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogal SS, Yan P, Rimland D, et al. Incidence and progression of chronic kidney disease after hepatitis C seroconversion: results from ERCHIVES. Dig Dis Sci. 2016;61:930‐936. [DOI] [PubMed] [Google Scholar]
- 10. Li DK, Ren Y, Fierer DS, et al. The short‐term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct‐acting antivirals: an ERCHIVES study. Hepatology. 2018;67:2244‐2253. [DOI] [PubMed] [Google Scholar]
- 11. Butt AA, Yan P, Shuaib A, Abou‐Samra AB, Shaikh OS, Freiberg MS. Direct‐acting antiviral therapy for HCV infection is associated with a reduced risk of cardiovascular disease events. Gastroenterology. 2019;156:987‐996. [DOI] [PubMed] [Google Scholar]
- 12. Butt AA, Yan P, Aslam S, Shaikh OS, Abou‐Samra AB. Hepatitis C virus (HCV) treatment with directly acting agents reduces the risk of incident diabetes: results from electronically retrieved cohort of HCV infected veterans (ERCHIVES). Clin Infect Dis. 2020;70:1153‐1160. [DOI] [PubMed] [Google Scholar]
- 13. Butt AA, Yan P, Shaikh OS, Lo Re V III, Abou‐Samra AB, Sherman KE. Treatment of HCV reduces viral hepatitis‐associated liver‐related mortality in patients: An ERCHIVES study. J Hepatol. 2020;73:277‐284. [DOI] [PubMed] [Google Scholar]
- 14. Iavarone M, D'Ambrosio R, Soria A, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol. 2020. 10.1016/j.jhep.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ibáñez‐Samaniego L, Bighelli F, Usón C, et al. Elevation of liver fibrosis index FIB‐4 is associated with poor clinical outcomes in patients with COVID‐19. J Infect Dis. 2020;222:726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bajaj JS, Garcia‐Tsao G, Biggins SW, et al. Comparison of mortality risk in patients with cirrhosis and COVID‐19 compared with patients with cirrhosis alone and COVID‐19 alone: multicentre matched cohort. Gut. 2020. 10.1136/gutjnl-2020-322118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarin SK, Choudhury A, Lau GK, et al. Pre‐existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID‐19 Liver Injury Spectrum Study). Hepatol Int. 2020. 10.1007/s12072-020-10072-8:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashemi N, Viveiros K, Redd WD, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID‐19: a multicentre United States experience. Liver Int. 2020. 10.1111/liv.14583 [DOI] [PMC free article] [PubMed] [Google Scholar]


