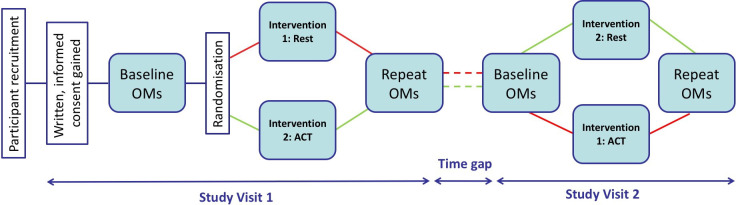
Figure 1.
Schematic of trial design. Diagram illustrating the research journey for a trial participant, from their recruitment and giving of informed consent to completing study day one: baseline outcome measures (OMs) followed by randomisation to intervention (rest or airway clearance therapy (ACT)) and postintervention OMs. The time gap in-between visits should be no longer than 3 months. On study day 2, the participant completes the same OMs before and after the other intervention. Participation in the study is then complete.