Abstract
Embryo implantation failure is considered a leading cause of infertility and a significant bottleneck for in vitro fertilization (IVF) treatment. Confirmed factors that lead to implantation failure involve unhealthy embryos, unreceptive endometrium, and asynchronous development and communication between the two. The quality of embryos is further dependent on sperm parameters, oocyte quality, and early embryo development after fertilization. The extensive involvement of such different factors contributes to the variability of implantation potential across different menstrual cycles. An ideal approach to predict the implantation outcome should not compromise embryo implantation. The use of clinical material, including follicular fluid, cumulus cells, sperm, seminal exosomes, spent blastocyst culture medium, blood, and uterine fluid, that can be collected relatively non-invasively without compromising embryo implantation in a transfer cycle opens new perspectives for the diagnosis of embryo implantation potential. Compositional comparison of these samples between fertile women and women or couples with implantation failure has identified both quantitative and qualitative differences in the expression of microRNAs (miRs) that hold diagnostic potential for implantation failure. Here, we review current findings of secreted miRs that have been identified to potentially be useful in predicting implantation outcome using material that can be collected relatively non-invasively. Developing non-invasive biomarkers of implantation potential would have a major impact on implantation failure and infertility.
Keywords: embryo implantation, non-invasive prediction, microRNAs, male factor, spent blastocyst culture medium, blood, uterine fluid, oocyte quality
Introduction
Infertility affects a staggering one in six couples worldwide (Wilcox et al., 1988) and can be a devastating condition for couples, with the failure to conceive recognized as a leading cause of psychological distress, depression, low self-esteem, and domestic violence (Chachamovich et al., 2010; Cui, 2010). A major contributor to infertility is the failure of blastocysts to implant, accounting for >50% of all failed pregnancies (Craciunas et al., 2019). While in vitro fertilization (IVF) has increasingly assisted couples to conceive, success rates have stagnated as still, ∼50% of good quality blastocysts fail to implant (Gardner and Balaban, 2016). Implantation is a highly complex biological process that requires the coordination between a healthy embryo and a receptive endometrium. The process is initiated via fertilization of a healthy oocyte, which occurs in the Fallopian tube. During fertilization, the female reproductive tract serves as a natural selection system to guarantee that the best quality sperm reaches and fertilizes the oocyte (Ralt et al., 1991). Once fertilized, the zygote travels through the Fallopian tube and develops to the morula stage when it reaches the uterine cavity. The morula stage embryo continues to develop to the blastocyst stage in the uterine cavity before implantation (Norwitz et al., 2001). This can take up to 72 h within which time the embryo and the endometrium communicate via secreted and cell surface factors to prepare for the initial adhesion and attachment (Ashary et al., 2018). Once the outer layer of the embryo, namely, the trophectoderm firmly attaches to the endometrial luminal epithelium, it initiates implantation. Failure of firm adhesion leads to implantation failure.
Successful implantation is based on the cumulative success of the above events. Implantation can be affected by many factors including sperm and oocyte quality, early development of the embryo, the endometrium, and the reciprocal communication between blastocysts and endometrium. During an IVF clinical setting, embryo quality is generally scored via assessment of morphology, expansion and hatching, development of inner cell mass, and the formation of the trophectoderm layer (Giorgetti et al., 1995; Gardner et al., 2000). The transfer of embryos graded as good or “transferable” can improve implantation and pregnancy outcome (Giorgetti et al., 1995; Gardner et al., 2000). However, these morphological criteria do not necessarily correlate with implantation potential. Embryos with similar morphologically good scores assessed to be of transferable quality from aged women (>38 years) have a significantly lower pregnancy rate compared to those of younger women (<38 years) (Giorgetti et al., 1995). It is estimated that overall, 50% of good quality embryos fail to implant (Gardner and Balaban, 2016). In addition to scores based on morphology, pre-implantation genetic testing is also used in some IVF clinics. This testing requires the collection of trophectoderm cells to assess the ploidy of blastocysts and can reveal one of the many characters that may affect implantation. Another clinical approach to improve implantation success is via the assessment of endometrial receptivity. A current clinical test, called endometrial receptivity array, is used to evaluate whether the endometrium is in phase or receptive (Díaz-Gimeno et al., 2011). However, this method is invasive as it requires an endometrial biopsy and does not diagnose a disrupted or dysregulated endometrium, and while promising, there is still a need to develop non-invasive methods to recognize a disrupted endometrium (Díaz-Gimeno et al., 2011). In addition to the endometrium, sperm quality also can affect implantation. Current clinical analysis of sperm quality relies on the basic assessments of sperm morphology and motility, which do not necessarily reflect their capability in facilitating embryo development and implantation.
Despite the available tests, abnormalities in sperm, oocytes, disrupted endometrium, and embryo–endometrial interactions that contribute to implantation failure are not able to be effectively determined, and implantation failure remains a significant bottleneck for IVF treatment. To improve this, emerging work focuses on assessing biomarkers in samples that can be collected relatively non-invasively and examining whether they reflect the implantation potential. While many different classes of potential biomarkers have been proposed, microRNAs (miRs) stand out as promising biomarkers to determine the quality of sperm, oocytes, embryos, and endometrium that could be used to predict implantation outcome. miRs are small non-coding RNAs that regulate gene expression and protein production (Bushati and Cohen, 2007). Secreted or extracellular miRs are highly stable in body fluids, reflect disease states, and are easily detectable in a short time frame making them highly suitable for biomarker detection (Cuman et al., 2015). Emerging evidence strongly suggests that miRs regulate human embryo implantation (Paul et al., 2019). Recent studies support their use as non-invasive biomarkers for sperm, oocyte, and blastocyst quality, endometrial receptivity, and blastocyst–endometrial interactions (Cuman et al., 2015; Machtinger et al., 2017; Li et al., 2019; Abu-Halima et al., 2020). This review aims to discuss the use of miRs for screening blastocyst quality and implantation potential, focusing on using human samples that can be collected relatively non-invasively.
Laboratory Identification of miRs With Translational Potential for Implantation Prediction
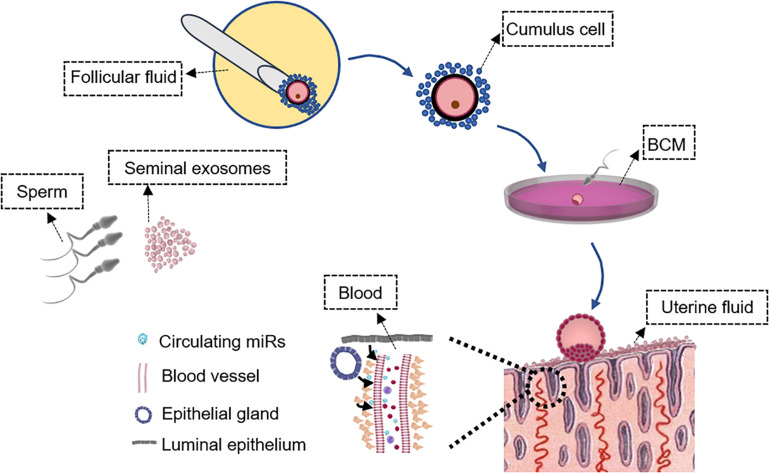
A standard IVF treatment broadly requires egg retrieval, sperm collection, IVF, and embryo culture before transfer. The collection of cumulus cells and follicular fluid is possible during egg retrieval without affecting IVF (Figure 1). Analysis of gene and miR expression in follicular fluid and cumulus cells indicates oocyte and embryo quality, thus the implantation potential from an embryo’s perspective (Hamel et al., 2008; Fu et al., 2018). Embryos are generally cultured up to 5–6 days to reach the blastocyst stage before transfer (Figure 1). Embryos secrete specific profiles of miRs that may reflect their quality and implantation potential (Kropp et al., 2014; Capalbo et al., 2016). The endometrial epithelium secretes factors into the uterine cavity to regulate implantation and uterine fluid, or uterine lavage washings can potentially be used to detect miRs as biomarkers for the prediction of receptivity and implantation (Boomsma et al., 2009). Blood contains extracellular miRs with expression levels of some miRs positively correlating with endometrial levels and can potentially indicate whether the endometrium is dysregulated or receptive (Kresowik et al., 2014). Abnormalities in sperm contribute to blastocyst development and quality (Yuan et al., 2016), which could impact implantation. It has been shown in mice that sperm relays epigenetic information to the oocyte during fertilization and influences pre-implantation embryo and offspring development (Sharma et al., 2016). The development of non-invasive biomarkers has driven extensive research in this area with an overall aim to improve the success rate of implantation and IVF treatment.
FIGURE 1.
Schematic of samples that can be collected non-invasively during in vitro fertilization (IVF) treatment. Characterization of the seminal exosome and sperm microRNA (miR) profiles could determine sperm quality to predict the potential of embryo development and IVF outcome. Analysis of miR expression in follicular fluid and cumulus cells may indicate oocyte and embryo quality, and implantation potential from an embryo’s perspective. miR profiles in BCM also likely reflect embryo quality that may not be distinguished by morphology-based assessment. Endometrial cells release miRs in the blood and the expression levels of at least some endometrial miRs are reflected in the blood. Assessment of circulating miRs in the blood may predict endometrial receptivity and implantation outcome. Uterine fluid miR levels reflect “local” endometrial miR secretion and can be collected without compromising embryo transfer to provide information on endometrial receptivity. These samples are outlined. BCM, blastocyst culture medium.
Cumulus Cells and Follicular Fluid
Cumulus cells are implicated in oocyte development and competence (Huang and Wells, 2010). In addition to interacting directly with the oocyte to facilitate maturation, cumulus cells are also bathed within the same follicular environment during oocyte maturation, thus may retain a footprint to reflect its quality and potential to form a viable embryo (Figure 1; Patrizio et al., 2007). It has been proven that cumulous cells are useful for non-invasive diagnosis of oocyte quality (Hamel et al., 2008; Devjak et al., 2016). Next-generation sequencing on human cumulus cells has revealed that miRs represent the major small RNA type, constituting as much as 71% of the total small RNAs (Xu et al., 2015). As a way of interaction, it has been shown that bovine cumulus cells and oocytes reciprocally affect the abundance of miRs in each cellular compartment (Abd El Naby, 2012), and these miRs readily control gene expression with extensive downstream functional implications. Gene expression studies on human cumulus cells have revealed transcripts that may be involved in oocyte maturation, implantation, and pregnancy with their regulatory miRs just beginning to be realized (Gasca et al., 2007; Hamel et al., 2008). During IVF treatment, cumulus cells are retrieved while still firmly attached to each oocyte, and as such, their collection can be sourced from individual oocytes and, thus provide an indication of the developmental potential for individual oocytes.
Follicular fluid is also collected during oocyte retrieval; however, unlike cumulus cells, follicular fluid normally collected during IVF stimulation is a pool from several oocytes, rather than a single oocyte to avoid multiple vaginal punctures. Despite this limitation, one study using pooled follicular fluid from individual patients has identified differences in miR expression between groups with different pregnancy outcomes (Scalici et al., 2016). This study screened five miRs and identified that hsa-miR-29a expression in the follicular fluid could predict pregnancy outcome with a specificity of 53.5% and has a higher discrimination power compared to prediction using embryo morphology scores (Scalici et al., 2016). Another two investigations collected follicular fluid from a single follicle and used microarrays to screen miRs that were able to predict the difference between good and bad quality blastocysts. Although hsa-miR-663b has been identified as a common miR that is inversely related to good quality blastocysts (Machtinger et al., 2017; Fu et al., 2018), the blastocyst quality discrimination method used in the two studies is based on routine morphological assessment. Therefore, the use of hsa-miR-663b as a marker of good quality blastocysts can similarly be determined by routine morphological assessment and likely provides limited application potential to predict embryo implantation outcome. In a clinical IVF setting, generally, multiple oocytes are retrieved at one time, and it is likely that they differ in quality and potential to develop into viable blastocysts. This may limit the extensive application of follicular fluid as it cannot be used to evaluate miRs released by individual oocytes.
Seminal Plasma and Sperm
Defective sperm function is widely acknowledged as a major contributor to infertility. Under physiological conditions, the sperm acquires functional competence during their transit through the epididymis and female reproductive tract. Both biophysical and biochemical changes occur along this journey, eventually culminating in the ability of sperm to undergo an acrosome reaction, recognize the oocyte, and contribute to embryo development (Zhou et al., 2018). A number of recent studies in mice have provided evidence that uptake of miRs from the epididymal luminal environment endows the sperm with the capability to contribute to the early embryonic development and, thus, implantation upon delivery to the oocyte (Yuan et al., 2016; Conine et al., 2018, 2019). miR profile comparisons between mouse caput and cauda sperm have identified 27 miRs that are specifically enriched in cauda sperm, compared to caput sperm (Nixon et al., 2015; Sharma et al., 2018). Microinjection of cauda sperm-enriched miRs into caput-derived embryos rescue gene expression defects before implantation in mice (Conine et al., 2019). Further investigation has identified an epididymosome-dependent mechanism for the selective delivery of miRs into the sperm during their transit in the epididymis in mice (Reilly et al., 2016; Trigg et al., 2019; Zhou et al., 2019). In humans, differences in miR expression profiles have been recorded in both seminal plasma and sperm relative to different embryo qualities and pregnancy outcomes (Mokánszki et al., 2019; Abu-Halima et al., 2020; Xu et al., 2020). miR sequencing analysis on sperm samples grouped according to different embryo qualities has identified higher expression levels of hsa-miR-191 in the sperm group with better embryo developmental outcome (Xu et al., 2020). hsa-miR-19b-3p has a lower expression in sperm that is associated with a successful pregnancy outcome (Abu-Halima et al., 2020). Another recent study selected 11 spermatogenesis-related miRs and revealed that hsa-let-7a, hsa-miR-7-1-3p, hsa-miR-141, hsa-miR-200a, and hsa-miR-429 were significantly elevated, while hsa-miR-15b, hsa-miR-34b, and hsa-miR-122 were significantly downregulated in both seminal plasma and sperm of infertile male patients with impaired sperm production, compared to males with normal fertility (Mokánszki et al., 2019). Seminal plasma has been identified with an enriched population of epididymosome-like vesicles, namely, seminal exosomes (Vojtech et al., 2014). A limitation of using seminal exosomes is that they represent a mixed population of extracellular vesicles originating not only from the epididymis but also from the prostate and seminal vesicles (Rolland et al., 2013), Whether miR profiles in these vesicles correlate with sperm quality requires investigation. Nevertheless, seminal exosomes have been implicated in the transfer of cargo to sperm, which promotes their motility, ability to capacitate, and complete acrosomal exocytosis, therefore, affecting sperm quality (Tompkins et al., 2015). In addition, exosomes isolated from seminal plasma can modulate the immune response and gene expression changes in the female reproductive tract (Robertson and Sharkey, 2016; Bai et al., 2018), which eventually facilitate implantation and pregnancy in humans. Such functions are at least mediated via seminal exosome-carried miRs (Machtinger et al., 2016), and like epididymosomes, seminal exosomes carry distinctive profiles of miRs (Vojtech et al., 2014). Improved characterization of the seminal plasma and sperm miR profiles could not only be beneficial in terms of uncovering the causative basis of male gamete dysfunction but also for the provision of urgently needed biomarkers of sperm quality to reliably predict the outcome of IVF treatments.
Spent Blastocyst Culture Medium
In an IVF setting, a fertilized oocyte is generally cultured in vitro for up to 5–6 days to the blastocyst stage before transfer. Spent culture media can be collected during media change without affecting embryo quality. It has been demonstrated that over 96% of miRs present in the spent culture media originate from the trophectoderm and can be consistently detected after blastulation under IVF culture conditions (Capalbo et al., 2016). It is tempting to speculate that blastocyst-secreted miRs participate in the regulation of trophectoderm–endometrial luminal epithelial interactions therefore implantation. In keeping with this notion, it has been identified that embryos with different implantation outcomes (implanted versus non-implanted) secrete different profiles of miR into the culture medium (Cuman et al., 2015; Borges et al., 2016; Capalbo et al., 2016). Increased expression of hsa-miR-142-3p and decreased expression of hsa-miR-20a and hsa-miR-30c have been identified in non-implanted blastocyst culture medium (BCM), compared to implanted BCM (Table 1; Borges et al., 2016; Capalbo et al., 2016). Further, microarray screens have identified a list of miRs exclusively detected in either implanted or non-implanted BCM (Table 1; Cuman et al., 2015; Capalbo et al., 2016). miR profiles in the BCM also likely reflect embryo quality and overall IVF outcome, as summarized in Table 1 (McCallie et al., 2010; Rosenbluth et al., 2014; Abu-Halima et al., 2020).
TABLE 1.
Identified microRNAs (miRs) in blastocyst culture medium (BCM) with diagnostic potential.
| Total number of miRs examined | miR expression | References |
| 12 miRs | BCM from polycystic ovaries: Hsa-let-7a ↓, hsa-miR-24 ↓, hsa-miR-92 ↓, hsa-miR-93 ↓, hsa-miR-19a ↓, hsa-miR-19b ↓ | McCallie et al., 2010 |
| 377 miRs | Non-implanted BCM: Hsa-miR-20a ↓, Hsa-miR-30c ↓ | Capalbo et al., 2016 |
| Only detected in implanted BCM: Hsa-miR-220, hsa-miR-146b-3p, hsa-miR-512-3p, hsa-miR-34c, hsa-miR-375 | ||
| 754 miRs | Failed IVF: Hsa-miR-191 ↑, hsa-miR-372 ↑, hsa-miR-645 ↑ | Rosenbluth et al., 2014 |
| 7 miRs | Non-implanted BCM: Hsa-miR-142-3p ↑ | Borges et al., 2016 |
| 784 miRs | Non-implanted group exclusively: Hsa-miR-374b-3p, hsa-miR-518c-3p, hsa-miR-126-3p, hsa-miR-361-5p, | Cuman et al., 2015 |
| hsa-miR-29b-2-5p, hsa-miR-516b-5p, hsa-miR-371a-5p, hsa-miR-372, hsa-miR-518a-3p, hsa-miR-149-5p, hsa-miR-571, | ||
| hsa-miR-943, hsa-miR-937-3p, hsa-miR-761, hsa-miR-106b-3p, hsa-miR-182-3p, hsa-miR-624, hsa-miR-661-5p, | ||
| hsa-miR-515-5p, hsa-let-7b-3p, hsa-miR-577, hsa-miR-1912 | ||
| Implanted group exclusively: Hsa-miR-23a-3p, hsa-miR-570-3p, hsa-miR-485-3p, hsa-miR-572, hsa-miR-26b-5p, | ||
| hsa-miR-150-5p, hsa-miR-744-5p, hsa-miR-874, hsa-miR-24-2-5p, hsa-miR-300, hsa-miR-619, hsa-miR-208a, | ||
| hsa-miR-612, hsa-miR-26b-3p, hsa-miR-632, hsa-miR-362-3p, hsa-miR-543, hsa-miR-380-5p, hsa-miR-638 | ||
| 372 miRs | High quality embryo: Hsa-miR-320a ↑, hsa-miR-15a-5p ↑, hsa-miR-21-5p ↓, hsa-miR-29a-3p ↓ | Abu-Halima et al., 2020 |
| Negative pregnancy: Hsa-let-7a-5p ↑, hsa-miR-19b-3p ↓ |
A previous study has proposed that while the pre-implantation embryo is in the uterine cavity, it packages regulatory miRs into extracellular vesicles (Ashary et al., 2018). They further propose that the packaged miRs are taken up by the endometrial luminal epithelial cells and alter their function to prepare for implantation. Incubation of primary human endometrial epithelial cells (HEECs) with BCM collected from embryos that were implanted increases their adhesive capacity to trophoblast cell line-formed spheroids (Cuman et al., 2013). Other notable examples include hsa-miR-661, which is exclusively secreted by blastocysts that fail to implant (Cuman et al., 2015). Secreted hsa-miR-661 from non-implanted BCM is taken up by HEECs and reduces their adhesion to trophoblast cell-formed spheroids (Cuman et al., 2015). A recent study also demonstrates that incubation of HEECs with BCM from embryos that implanted, compared to embryos that did not implant during IVF, leads to a substantial change in the expression of long non-coding RNAs in the HEECs (Takamura et al., 2019). PTENP1 is one of the most decreased long non-coding RNAs in HEECs after being treated with BCM from embryos that fail to implant (Takamura et al., 2019). Functionally, knockdown of PTENP1 impairs HEEC adhesion via a miR-dependent mechanism to downregulate gene targets essential for receptivity (Takamura et al., 2019).
The implanted and non-implanted embryos from which BCM was collected had an indistinguishable morphology based on currently available assessment of embryo quality. Thus, miRs in the BCM may serve as promising non-invasive biomarkers to improve the diagnostic accuracy of embryo quality and implantation potential. An obvious challenge to achieve this is to determine which cohorts of miRs are present in BCM samples that correlate with implantation outcome, in particular, regardless of embryo culture conditions. Although some miRs such as hsa-miR-19b-3p and hsa-miR-372 have been identified in at least two independent studies (Table 1), the comparison of secreted miRs in BCM with different implantation outcomes has demonstrated a generally inconsistent result among different studies (McCallie et al., 2010; Rosenbluth et al., 2014; Cuman et al., 2015; Borges et al., 2016; Capalbo et al., 2016; Abu-Halima et al., 2020). Contributing factors to this inconsistency include diverse embryo culture conditions in different IVF clinics, unstandardized protocols, manual effects on RNA isolation, and miR detection (Belandres et al., 2019). In addition, an obvious confounder of associating miRs in BCM with failed implantation outcome is the potential effects of the endometrium. A failed implantation group from which BCM was collected could be due to poor embryo quality, dysregulated endometrium, or altered receptivity window. The communication between an embryo and endometrium remains a “black box,” and it is perhaps notable that not all secreted miRs are taken up by endometrial luminal epithelial cells to regulate implantation. For future diagnostic purposes, it is necessary to identify which miRs are taken up by endometrium and their actions on the endometrium. A panel of miRs will likely to be included, with the functional consequence of each individual miR on implantation being confirmed in ideally both humans (in vitro) and preclinical animal models (in vivo). Detailed functional studies have only covered a small proportion of miRs identified so far (Table 2; Chu et al., 2015; Cuman et al., 2015; Kang et al., 2015; Kottawatta et al., 2015; Vilella et al., 2015; Zhang et al., 2015; Cai et al., 2016; Chen et al., 2016; Zheng et al., 2017; Sirohi et al., 2018; Winship et al., 2018; Balaguer et al., 2019; Griffiths et al., 2019; Takamura et al., 2019).
TABLE 2.
miRs that regulate embryo implantation.
| miR | Phenotype | Target (s) | Affect implantation in animal model | References |
| Hsa-miR-200c | Overexpression of miR-200c in RL95-2 and Ishikawa cells impair receptive ability in vitro | FUT4↓ | Yes (mouse) | Zheng et al., 2017 |
| Hsa-miR-661 | Secreted by non-implanted embryo and transferred to HEECs to affect their adhesive capacity in vitro | PVRL1↓ | Not available | Cuman et al., 2015 |
| Overexpression of miR-661 in HEECs in vitro impairs adhesion | MDM2↓ | Not available | Winship et al., 2018 | |
| Hsa-miR-181a | Inhibition of miR-181a compromises human endometrial stromal cell decidualization in vitro | KLF12↓ | Yes (mouse) | Chu et al., 2015; Zhang et al., 2015 |
| Hsa-miR-212 | hCG stimulates miR-212 expression in Ishikawa cells to favor spheroid adhesion in vitro | OLFM1↓, CTBP1↓ | Not available | Kottawatta et al., 2015 |
| Hsa-miR-145 | Overexpression of miR-145 in Ishikawa cells affects mouse embryo attachment in vitro | IGF1R↓ | Not available | Kang et al., 2015 |
| Hsa-miR-30d | Human endometrial secreted miR-30d is taken up by mouse pre-implantation embryo and increases embryo adhesion in vitro | Itgb3↑, Itga7↑, Cdh5↑ | Yes (Mouse) | Vilella et al., 2015; Balaguer et al., 2019 |
| Overexpression of miR-30d in Ishikawa cells facilitates adhesion in vitro | SNAI1↓ | Yes (Mouse) | Cai et al., 2016; Balaguer et al., 2019 | |
| Hsa-miR-125b | Overexpression of miR-125b in HEECs inhibits cell migration and invasion in vitro | MMP26↓ | Yes (Mouse) | Chen et al., 2016 |
| Hsa-miR-29c | Overexpression of miR-29c in HEECs impairs adhesion in vitro | COL4A1↓ | Not available | Griffiths et al., 2019 |
| Hsa-miR-590-3p | Overexpression of miR-590-3p in HEECs in vitro impairs adhesion | N/A | Not available | Takamura et al., 2019 |
| Hsa-miR-140 | Overexpression of miR-140 in RL95-2 endometrial epithelial cells impairs adhesion and spheroid outgrowth in vitro | N/A | Yes (Rat) | Sirohi et al., 2018 |
Blood
MicroRNAs are also readily secreted into the blood. Circulating miRs are packaged in membrane-bound vesicles, attached to high-density lipoproteins or bound to RNA-binding proteins, which endow them with striking stability in the blood (Schwarzenbach et al., 2014). The human endometrium features a rich blood supply with the responsibility to provide an optimal environment to promote receptivity and implantation (Farrer-Brown et al., 1970). Endometrial cells may secrete/transport a number of miRs to the tissue site of action by way of the blood, and studies suggest that endometrial expression levels of at least some miRs are reflected in the blood (Kresowik et al., 2014; Di Pietro et al., 2018). Circulating miRs in the blood may be able to predict endometrial receptivity and implantation. A previous study used whole blood and paired mid-secretory phase endometrial tissue to determine whether circulating miRs could distinguish fertile from recurrent implantation failure patients (Rekker et al., 2018). miR-30a-5p was identified as differentially expressed in whole blood between the two groups; however, this difference was not reflected in the paired endometrial tissue (Rekker et al., 2018). One possible explanation is that blood cells express miRs (Jickling et al., 2014), which may mask endometrial tissue-secreted miRs. Recent work using paired serum and mid-secretory phase endometrium investigated five miRs and identified a positive correlation of hsa-miR-31 expression levels between serum and endometrial tissue (Kresowik et al., 2014). Alternatively, extracellular miR expression levels do not necessarily reflect cellular expression levels. Whether miR biomarkers have critical functions in endometrial receptivity also needs to be determined experimentally, as differentially expressed circulating miRs between women with normal fertility and infertility may not all have functional relevance in receptivity or implantation. Functional studies of the identified circulating and cellular miRs in receptivity and implantation models could provide evidence to support their potential application as biomarkers and treatment targets. For example, hsa-miR-200c expression is increased in the serum of infertility and abortion patients, compared to healthy women (Zheng et al., 2017). Functional analysis using both human endometrial cell lines and a mouse model demonstrates that hsa-miR-200c overexpression impairs endometrial cell receptivity in both species (Zheng et al., 2017).
The obvious challenge to predict implantation outcome using miRs in the blood will be to distinguish the endometrial secreted miRs from miRs secreted by other tissues. Of note, the process of embryo implantation somewhat resembles that of cancer cell metastasis. Both processes share some of the cellular mechanisms in cell adhesion, invasion, and angiogenesis (Murray and Lessey, 1999). miRs such as hsa-miR-29c (Griffiths et al., 2019) and hsa-miR-125b (Chen et al., 2016) that are dysregulated in the endometrium from infertile women are also associated with gastric and endometrial cancers (Shang et al., 2012; Wang et al., 2019). Cancer cells releasing miRs into the blood may confound the detection of miRs secreted by the endometrium. In this regard, an important feature of the endometrium is that it regenerates itself at each menstrual cycle. The endometrium is only receptive to an implanting embryo within a very short window in the mid-secretory phase (Ashary et al., 2018). Such a functional switch is mediated by coordinated changes of miR expression (Vilella et al., 2015). These phase-dependent changes, in turn, may endow endometrial-secreted miRs with unique cycle-dependent expression fingerprints that can be used to distinguish from the background of other potential tissue-secreted miRs. This theory is evidenced by a previous study comparing miR expression between the proliferative phase and mid-secretory phase in paired serum and endometrial tissue from fertile women. hsa-miR-31 has been identified as a potential biomarker that is elevated in both serum and endometrium in the mid-secretory phase, compared to the proliferative phase (Kresowik et al., 2014). It is also essential to investigate appropriate controls from different pathologies as comparative groups. The predictive application of blood miRs on implantation will likely be based on the multiple measurements of miR expression at different phases within a menstrual cycle.
Another challenge of using miR levels in the blood for biomarker purposes has been identified in cancer diagnosis. A previous study selected 79 solid cancer-circulating miR biomarkers and determined their expression levels in blood cells. Forty-six of the 79 miRs were highly expressed in the blood cells (Pritchard et al., 2012). Plasma isolated from the blood with different blood cell counts or hemolysis impacted the expression levels of select miRs (Pritchard et al., 2012). Inconsistency has also been observed between plasma and serum levels of miR between pregnant and non-pregnant patient groups after embryo transfer (Yang et al., 2018). To improve the accuracy of prediction, a panel of miRs is required, as has been proposed for cancer diagnosis (Madhavan et al., 2013). To achieve this, an investigation of miR levels from large cohorts of women with different etiologies of infertility and other pathologies is required for their potential use as biomarkers. We have previously identified a dysregulation of miR-processing machinery in the endometrium of a cohort of infertile patients, which would have an overall impact on miR secretion due to compromised miR processing within the cell (Loke et al., 2019). The miR secretion in this cohort may be different compared to other infertile cohorts caused by different etiologies. Identifying which miRs are responsible for ensuring endometrial receptivity is also required to determine whether the biomarkers may also be useful as treatment targets of dysregulated endometrial receptivity.
Uterine Fluid
The uterine fluid is secreted by the human endometrium as an indirect approach to communicate with an embryo for the preparation of implantation. Compared to other body fluids, uterine fluid is a more “local” secretion and, thus, may provide direct information when assessing biomarkers for implantation. Detailed compositional analysis has revealed that uterine fluid contains miRs and proteins with changed profiles across the menstrual cycle (Scotchie et al., 2009; Ng et al., 2013). Functional analysis has proven that endometrial cells secreted miRs, such as hsa-miR-30d, that are taken up by the embryo via the trophectoderm and regulate adhesion in vitro (Vilella et al., 2015). Further investigation demonstrates that secreted miRs in the uterine fluid target an extensive of implantation-related genes (Ng et al., 2013). Of note, the miRs in the uterine fluid can be sourced from different endometrial cells and the blood. This can be determined via in situ hybridization on endometrial sections, like what has been done for protein via immunostaining (Hannan et al., 2010). Uterine fluid can be collected via either aspiration or lavage without compromising implantation (Hannan et al., 2012). To the best of our knowledge, however, most currently available studies on uterine fluid have focused on comparing the proteins between fertile and infertile patients (Hannan et al., 2010; Salamonsen et al., 2013). There are presently limited studies investigating the potential of using miRs in the uterine fluid as a diagnostic approach for implantation.
Overall Challenges of Using Secreted miRs to Predict Implantation
Although miRs are highly desirable as non-invasive biomarkers to predict implantation, this field of research is somewhat confounded by a general inconsistency of miR expression levels across different studies. It has been identified that a number of factors including RNA isolation and detection systems can contribute to this inconsistency. Recently published work from one laboratory, which used different commercial kits to isolate RNA, demonstrated that the recovery of RNA was variable between the commercial kits (El-Khoury et al., 2016; Wright et al., 2020). In addition, the selection of endogenous controls to normalize the target miR expression levels directly affects the results, and such importance has been neglected by some studies. For miR normalization, an ideal endogenous control should be stably expressed in the body fluid with minimal biological variation, and the expression should not change with different implantation outcomes. It is known that in body fluids, the expression of some cellular endogenous controls may vary between different samples bringing deviation in normalization. It is an essential first step to compare the expression variability of a number of endogenous control candidates in a given body fluid system and confirm their stability. This has not been conducted in some studies and may have contributed to the variability of miR expression. A workflow has been proposed to identify the best normalization control (Schwarzenbach et al., 2015). All these steps introducing impact factors require standardization before a solid conclusion can be drawn.
Adding to this challenge is the observation that inherent differences between women, together with different IVF protocols, may lead to differential expression patterns of miR in the human endometrium. A microarray study has identified that luteal support following controlled ovarian stimulation has a profound influence on the miR profile in the endometrium (Zhao et al., 2012). Specifically, progesterone supplementation is associated with a significant increase in miR expression in the endometrium compared to a no steroid supplementation group following controlled ovarian stimulation (Zhao et al., 2012). The findings are in accordance with a previous report identifying differential expression patterns of miR between natural and stimulated IVF cycles (Sha et al., 2011). In addition, patients receiving the same IVF treatment who have different serum progesterone levels have been identified to have different miR expression patterns in the endometrial tissue collected 6 days after oocyte retrieval (Li et al., 2011). Microarray analysis of the endometrium identified four miRs (hsa-miR-451, hsa-miR-424, hsa-miR-125b, and hsa-miR-30b) that were decreased in the high serum progesterone group (Li et al., 2011). The effects of controlled ovarian stimulation and luteal phase support need to be considered when comparing data from different studies.
Conclusion
Measurement of miRs in the samples that can be collected without compromising embryo transfer in the same menstrual cycle opens new perspectives for the diagnosis of embryo implantation potential. Unfortunately, our understanding of the mechanisms of how miR dysregulation impacts implantation and how this accordingly affects miR secretion remains far from complete. Interpretation of research findings is confounded by unstandardized assessment of miRs in a given body fluid. Resolving these questions would have a major impact on biomarker development and clinical practice for reproductive clinicians and scientists. This includes optimizing the selection of embryos for transfer during IVF, improvement of implantation success rates, and the minimization of multiple pregnancies. It is likely that a combination of samples that can be collected either non-invasively or relatively non-invasively, as summarized in this review, will be useful to assess implantation potential at different stages of conceptus establishment and development. This relies on research to find miR biomarkers related to implantation regulation and the development of new technologies to improve miR detection. A few microfluidic devices have been developed recently with a larger capacity to include more miRs and reduce analysis time. Improved diagnosis of embryo implantation could have a profound effect on psychological and financial well-being on women and couples undergoing IVF treatment.
Author Contributions
Both authors made substantial contributions to the conception of this review and the critical appraisal of the literature summarized herein, wrote the manuscript, and approved the final version of this article before submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by a project grant (APP1120689) and a senior research fellowship (#550905) from the National Health and Medical Research Council (NHMRC) of Australia to ED. WZ was supported by an Early Career Researcher Grant and a Department of Obstetrics and Gynaecology Innovation Grant (The University of Melbourne).
References
- Abd El Naby W. S. H. (2012). Expression analysis of regulatory MicroRNA in bovine cumulus oocyte complex and preimplantation embryos. Zygote 21 31–51. 10.1017/s0967199411000566 [DOI] [PubMed] [Google Scholar]
- Abu-Halima M., Khaizaran Z. A., Ayesh B. M., Fischer U., Khaizaran S. A., Al-Battah F., et al. (2020). MicroRNAs in combined spent culture media and sperm are associated with embryo quality and pregnancy outcome. Fertil. Steril. 113 970.e2–980.e2. [DOI] [PubMed] [Google Scholar]
- Ashary N., Tiwari A., Modi D. (2018). Embryo implantation: war in times of love. Endocrinology 159 1188–1198. 10.1210/en.2017-03082 [DOI] [PubMed] [Google Scholar]
- Bai R., Latifi Z., Kusama K., Nakamura K., Shimada M., Imakawa K. (2018). Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem. Biophys. Res. Commun. 495 1094–1101. 10.1016/j.bbrc.2017.11.100 [DOI] [PubMed] [Google Scholar]
- Balaguer N., Moreno I., Herrero M., Gonzáléz-Monfort M., Vilella F., Simón C. (2019). MicroRNA-30d deficiency during preconception affects endometrial receptivity by decreasing implantation rates and impairing fetal growth. Am. J. Obstet. Gynecol. 221 46.e1–46.e16. [DOI] [PubMed] [Google Scholar]
- Belandres D., Shamonki M., Arrach N. (2019). Current status of spent embryo media research for preimplantation genetic testing. J. Assist. Reprod. Genet. 36 819–826. 10.1007/s10815-019-01437-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma C., Kavelaars A., Eijkemans M., Lentjes E., Fauser B., Heijnen C., et al. (2009). Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. 24 1427–1435. 10.1093/humrep/dep011 [DOI] [PubMed] [Google Scholar]
- Borges E., Jr., Setti A. S., Braga D. P., Geraldo M. V., Figueira R. D. C. S., Iaconelli A., Jr. (2016). miR-142-3p as a biomarker of blastocyst implantation failure-A pilot study. JBRA Assist. Reprod. 20 200–205. 10.5935/1518-0557.20160039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N., Cohen S. M. (2007). microRNA functions. Annu. Rev. Cell Dev. Biol. 23 175–205. [DOI] [PubMed] [Google Scholar]
- Cai J. L., Liu L. L., Hu Y., Jiang X. M., Qiu H. L., Sha A.-G., et al. (2016). Polychlorinated biphenyls impair endometrial receptivity in vitro via regulating mir-30d expression and epithelial mesenchymal transition. Toxicology 365 25–34. 10.1016/j.tox.2016.07.017 [DOI] [PubMed] [Google Scholar]
- Capalbo A., Ubaldi F. M., Cimadomo D., Noli L., Khalaf Y., Farcomeni A., et al. (2016). MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil. Steril. 105 225–235. 10.1016/j.fertnstert.2015.09.014 [DOI] [PubMed] [Google Scholar]
- Chachamovich J. R., Chachamovich E., Ezer H., Fleck M. P., Knauth D., Passos E. P. (2010). Investigating quality of life and health-related quality of life in infertility: a systematic review. J. Psychosom. Obstet. Gynaecol. 31 101–110. 10.3109/0167482x.2010.481337 [DOI] [PubMed] [Google Scholar]
- Chen C., Zhao Y., Yu Y., Li R., Qiao J. (2016). MiR-125b regulates endometrial receptivity by targeting MMP26 in women undergoing IVF-ET with elevated progesterone on HCG priming day. Sci. Rep. 6:25302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B., Zhong L., Dou S., Wang J., Li J., Wang M., et al. (2015). miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor. J. Mol. Cell Biol. 7 12–22. 10.1093/jmcb/mjv006 [DOI] [PubMed] [Google Scholar]
- Conine C. C., Sun F., Song L., Rivera-Pérez J. A., Rando O. J. (2018). Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell 46 470–480. 10.1016/j.devcel.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine C. C., Sun F., Song L., Rivera-Pérez J. A., Rando O. J. (2019). MicroRNAs absent in caput sperm are required for normal embryonic development. Dev. Cell 50 7–8. 10.1016/j.devcel.2019.06.007 [DOI] [PubMed] [Google Scholar]
- Craciunas L., Gallos I., Chu J., Bourne T., Quenby S., Brosens J. J., et al. (2019). Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum. Reprod. Upd. 25 202–223. 10.1093/humupd/dmy044 [DOI] [PubMed] [Google Scholar]
- Cui W. (2010). Mother or nothing: the agony of infertility. Bull. World Health Organ. 88 881–882. 10.2471/blt.10.011210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuman C., Menkhorst E., Rombauts L., Holden S., Webster D., Bilandzic M., et al. (2013). Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success. Hum. Reprod. 28 1161–1171. 10.1093/humrep/det058 [DOI] [PubMed] [Google Scholar]
- Cuman C., Van Sinderen M., Gantier M. P., Rainczuk K., Sorby K., Rombauts L., et al. (2015). Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. eBio Med. 2 1528–1535. 10.1016/j.ebiom.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devjak R., Papler T. B., Verdenik I., Tacer K. F., Bokal E. V. (2016). Embryo quality predictive models based on cumulus cells gene expression. Balkan J. Med. Genet. 19 5–12. 10.1515/bjmg-2016-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro C., Caruso S., Battaglia R., Iraci Sareri M., La Ferlita A., Strino F., et al. (2018). MiR-27a-3p and miR-124-3p, upregulated in endometrium and serum from women affected by Chronic Endometritis, are new potential molecular markers of endometrial receptivity. Am. J. Reprod. Immunol. 80:e12858. 10.1111/aji.12858 [DOI] [PubMed] [Google Scholar]
- Díaz-Gimeno P., Horcajadas J. A., Martínez-Conejero J. A., Esteban F. J., Alamá P., Pellicer A., et al. (2011). A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 95 50–60. 10.1016/j.fertnstert.2010.04.063 [DOI] [PubMed] [Google Scholar]
- El-Khoury V., Pierson S., Kaoma T., Bernardin F., Berchem G. (2016). Assessing cellular and circulating miRNA recovery: the impact of the RNA isolation method and the quantity of input material. Sci. Rep. 6:19529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer-Brown G., Beilby J., Tarbit M. (1970). The blood supply of the uterus: 1. Arterial vasculature. J. Obstet. Gynaecol. Br. Common. 77 673–681. 10.1111/j.1471-0528.1970.tb03592.x [DOI] [PubMed] [Google Scholar]
- Fu J., Qu R. G., Zhang Y. J., Gu R. H., Li X., Sun Y. J., et al. (2018). Screening of miRNAs in human follicular fluid reveals an inverse relationship between microRNA-663b expression and blastocyst formation. Reprod. Biomed. Online 37 25–32. 10.1016/j.rbmo.2018.03.021 [DOI] [PubMed] [Google Scholar]
- Gardner D. K., Balaban B. (2016). Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and ‘OMICS’: is looking good still important? Mol. Hum. Reprod. 22 704–718. 10.1093/molehr/gaw057 [DOI] [PubMed] [Google Scholar]
- Gardner D. K., Lane M., Stevens J., Schlenker T., Schoolcraft W. B. (2000). Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil. Steril. 73 1155–1158. 10.1016/s0015-0282(00)00518-5 [DOI] [PubMed] [Google Scholar]
- Gasca S., Pellestor F., Assou S., Loup V., Anahory T., Dechaud H., et al. (2007). Identifying new human oocyte marker genes: a microarray approach. Reprod. Biomed. Online 14 175–183. 10.1016/s1472-6483(10)60785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti C., Terriou P., Auquier P., Hans E., Spach J. L., Salzmann J., et al. (1995). Implantation: embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum. Reprod. 10 2427–2431. 10.1093/oxfordjournals.humrep.a136312 [DOI] [PubMed] [Google Scholar]
- Griffiths M., Van Sinderen M., Rainczuk K., Dimitriadis E. (2019). miR-29c overexpression and COL4A1 downregulation in infertile human endometrium reduces endometrial epithelial cell adhesive capacity in vitro implying roles in receptivity. Sci. Rep. 9:8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel M., Dufort I., Robert C., Gravel C., Leveille M. C., Leader A., et al. (2008). Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum. Reprod. 23 1118–1127. 10.1093/humrep/den048 [DOI] [PubMed] [Google Scholar]
- Hannan N. J., Nie G., Rainzcuk A., Rombauts L., Salamonsen L. A. (2012). Uterine lavage or aspirate: which view of the intrauterine environment? Reprod. Sci. 19 1125–1132. 10.1177/1933719112443879 [DOI] [PubMed] [Google Scholar]
- Hannan N. J., Stephens A. N., Rainczuk A., Hincks C., Rombauts L. J., Salamonsen L. A. (2010). 2D-DiGE analysis of the human endometrial secretome reveals differences between receptive and nonreceptive states in fertile and infertile women. J. Proteome Res. 9 6256–6264. 10.1021/pr1004828 [DOI] [PubMed] [Google Scholar]
- Huang Z., Wells D. (2010). The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol. Hum. Reprod. 16 715–725. 10.1093/molehr/gaq031 [DOI] [PubMed] [Google Scholar]
- Jickling G. C., Ander B. P., Zhan X., Noblett D., Stamova B., Liu D. (2014). microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One 9:e99283. 10.1371/journal.pone.0099283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. J., Lees M., Matthews L. C., Kimber S. J., Forbes K., Aplin J. D. (2015). miR-145 suppresses embryo–epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J. Cell Sci. 128 804–814. 10.1242/jcs.164004 [DOI] [PubMed] [Google Scholar]
- Kottawatta K. S., So K. H., Kodithuwakku S. P., Ng E. H., Yeung W. S., Lee K. F. (2015). MicroRNA-212 regulates the expression of olfactomedin 1 and C-terminal binding protein 1 in human endometrial epithelial cells to enhance spheroid attachment in vitro. Biol. Reprod. 93:109. [DOI] [PubMed] [Google Scholar]
- Kresowik J. D., Devor E. J., Van Voorhis B. J., Leslie K. K. (2014). MicroRNA-31 is significantly elevated in both human endometrium and serum during the window of implantation: a potential biomarker for optimum receptivity. Biol. Reprod. 91:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp J., Salih S. M., Khatib H. (2014). Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front Genet 5:91. 10.3389/fgene.2014.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Qiao J., Wang L., Li L., Zhen X., Liu P., et al. (2011). MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod. Biol. Endocrinol. 9:29. 10.1186/1477-7827-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Greenblatt E., Chan C. (2019). Isolation and profiling of extracellular vesicles in uterine fluid to determine novel markers of endometrial receptivity. Fertil. Steril. 112:e314 10.1016/j.fertnstert.2019.07.911 [DOI] [PubMed] [Google Scholar]
- Loke H., Rainczuk K., Dimitriadis E. (2019). MicroRNA biogenesis machinery is dysregulated in the endometrium of infertile women suggesting a role in receptivity and infertility. J. Histochem. Cytochem. 67 589–599. 10.1369/0022155419854064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R., Laurent L. C., Baccarelli A. A. (2016). Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Upd. 22 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R., Rodosthenous R. S., Adir M., Mansour A., Racowsky C., Baccarelli A. A., et al. (2017). Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J. Assist. Reprod. Genet. 34 525–533. 10.1007/s10815-017-0876-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan D., Cuk K., Burwinkel B., Yang R. (2013). Cancer diagnosis and prognosis decoded by blood-based circulating microRNA signatures. Front. Genet. 4:116. 10.3389/fgene.2013.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallie B., Schoolcraft W. B., Katz-Jaffe M. G. (2010). Aberration of blastocyst microRNA expression is associated with human infertility. Fertil. Steril. 93 2374–2382. 10.1016/j.fertnstert.2009.01.069 [DOI] [PubMed] [Google Scholar]
- Mokánszki A., Molnár Z., Varga Tóthné E., Bodnár B., Jakab A., Bálint B. L., et al. (2019). Altered microRNAs expression levels of sperm and seminal plasma in patients with infertile ejaculates compared with normozoospermic males. Hum. Fertil. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Lessey B. A. (1999). Embryo Implantation, and Tumor Metastasis: Common Pathways of Invasion, and Angiogenesis, Semin Reprod Endocrinol. New York, NY: Thieme Medical Publishers, 275–290. [DOI] [PubMed] [Google Scholar]
- Ng Y. H., Rome S., Jalabert A., Forterre A., Singh H., Hincks C. L., et al. (2013). Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 8:e58502. 10.1371/journal.pone.0058502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B., Stanger S. J., Mihalas B. P., Reilly J. N., Anderson A. L., Tyagi S., et al. (2015). The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 93:91. [DOI] [PubMed] [Google Scholar]
- Norwitz E. R., Schust D. J., Fisher S. J. (2001). Implantation and the survival of early pregnancy. N. Engl. J. Med. 345 1400–1408. 10.1056/nejmra000763 [DOI] [PubMed] [Google Scholar]
- Patrizio P., Fragouli E., Bianchi V., Borini A., Wells D. (2007). Molecular methods for selection of the ideal oocyte. Reprod. Biomed. Online 15 346–353. 10.1016/s1472-6483(10)60349-5 [DOI] [PubMed] [Google Scholar]
- Paul A. B., Sadek S. T., Mahesan A. M. (2019). The role of microRNAs in human embryo implantation: a review. J. Assist. Reprod. Genet. 36 179–187. 10.1007/s10815-018-1326-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C. C., Kroh E., Wood B., Arroyo J. D., Dougherty K. J., Miyaji M. M., et al. (2012). Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev. Res. 5 492–497. 10.1158/1940-6207.capr-11-0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralt D., Goldenberg M., Fetterolf P., Thompson D., Dor J., Mashiach S., et al. (1991). Sperm attraction to a follicular factor (s) correlates with human egg fertilizability. Proc. Natl. Acad. Sci. U.S.A. 88 2840–2844. 10.1073/pnas.88.7.2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. N., McLaughlin E. A., Stanger S. J., Anderson A. L., Hutcheon K., Church K., et al. (2016). Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 6 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekker K., Altmäe S., Suhorutshenko M., Peters M., Martinez-Blanch J. F., Codoñer F. M., et al. (2018). A two-cohort RNA-seq study reveals changes in endometrial and blood miRNome in fertile and infertile women. Genes 9:574. 10.3390/genes9120574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. A., Sharkey D. J. (2016). Seminal fluid and fertility in women. Fertil. Steril. 106 511–519. 10.1016/j.fertnstert.2016.07.1101 [DOI] [PubMed] [Google Scholar]
- Rolland A. D., Lavigne R., Dauly C., Calvel P., Kervarrec C., Freour T., et al. (2013). Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum. Reprod. 28 199–209. 10.1093/humrep/des360 [DOI] [PubMed] [Google Scholar]
- Rosenbluth E. M., Shelton D. N., Wells L. M., Sparks A. E., Van Voorhis B. J. (2014). Human embryos secrete microRNAs into culture media—a potential biomarker for implantation. Fertil. Steril. 101 1493–1500. 10.1016/j.fertnstert.2014.01.058 [DOI] [PubMed] [Google Scholar]
- Salamonsen L. A., Edgell T., Rombauts L. J., Stephens A. N., Robertson D. M., Rainczuk A., et al. (2013). Proteomics of the human endometrium and uterine fluid: a pathway to biomarker discovery. Fertil. Steril. 99 1086–1092. 10.1016/j.fertnstert.2012.09.013 [DOI] [PubMed] [Google Scholar]
- Scalici E., Traver S., Mullet T., Molinari N., Ferrieres A., Brunet C., et al. (2016). Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci. Rep. 6:24976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach H., Da Silva A. M., Calin G., Pantel K. (2015). Data normalization strategies for microRNA quantification. Clin. Chem. 61 1333–1342. 10.1373/clinchem.2015.239459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach H., Nishida N., Calin G. A., Pantel K. (2014). Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 11 145–156. 10.1038/nrclinonc.2014.5 [DOI] [PubMed] [Google Scholar]
- Scotchie J. G., Fritz M. A., Mocanu M., Lessey B. A., Young S. L. (2009). Proteomic analysis of the luteal endometrial secretome. Reprod. Sci. 16 883–893. 10.1177/1933719109337165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha A. G., Liu J. L., Jiang X. M., Ren J. Z., Ma C. H., Lei W., et al. (2011). Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil. Steril. 96 150–155. 10.1016/j.fertnstert.2011.04.072 [DOI] [PubMed] [Google Scholar]
- Shang C., Lu Y. M., Meng L. R. (2012). MicroRNA-125b down-regulation mediates endometrial cancer invasion by targeting ERBB2. Med. Sci. Monit. 18 BR149–BR155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U., Conine C. C., Shea J. M., Boskovic A., Derr A. G., Bing X. Y., et al. (2016). Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351 391–396. 10.1126/science.aad6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U., Sun F., Conine C. C., Reichholf B., Kukreja S., Herzog V. A., et al. (2018). Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev. Cell 46 481–494. 10.1016/j.devcel.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi V. K., Gupta K., Kumar R., Shukla V., Dwivedi A. (2018). Selective estrogen receptor modulator Ormeloxifene suppresses embryo implantation via inducing miR-140 and targeting insulin-like growth factor 1 receptor in rat uterus. J. Steroid Biochem. Mol. Biol. 178 272–282. 10.1016/j.jsbmb.2018.01.006 [DOI] [PubMed] [Google Scholar]
- Takamura M., Zhou W., Rombauts L., Dimitriadis E. (2019). The long noncoding RNA PTENP1 regulates human endometrial epithelial adhesive capacity in vitro: implications in infertility. Biol. Reprod. 102 53–62. 10.1093/biolre/ioz173 [DOI] [PubMed] [Google Scholar]
- Tompkins A. J., Chatterjee D., Maddox M., Wang J., Arciero E., Camussi G., et al. (2015). The emergence of extracellular vesicles in urology: fertility, cancer, biomarkers and targeted pharmacotherapy. J. Extracell. Vesicles 4:23815. 10.3402/jev.v4.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigg N. A., Eamens A. L., Nixon B. (2019). The contribution of epididymosomes to the sperm small RNA profile. Reproduction 157 R209–R223. [DOI] [PubMed] [Google Scholar]
- Vilella F., Moreno-Moya J. M., Balaguer N., Grasso A., Herrero M., Martínez S., et al. (2015). Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 142 3210–3221. 10.1242/dev.124289 [DOI] [PubMed] [Google Scholar]
- Vojtech L., Woo S., Hughes S., Levy C., Ballweber L., Sauteraud R. P., et al. (2014). Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42 7290–7304. 10.1093/nar/gku347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yu T., Li W., Li M., Zuo Q., Zou Q., et al. (2019). The miR-29c-KIAA1199 axis regulates gastric cancer migration by binding with WBP11 and PTP4A3. Oncogene 38 3134–3150. 10.1038/s41388-018-0642-0 [DOI] [PubMed] [Google Scholar]
- Wilcox A. J., Weinberg C. R., O’Connor J. F., Baird D. D., Schlatterer J. P., Canfield R. E., et al. (1988). Incidence of early loss of pregnancy. N. Engl. J. Med. 319 189–194. [DOI] [PubMed] [Google Scholar]
- Winship A., Ton A., Van Sinderen M., Menkhorst E., Rainczuk K., Griffiths M., et al. (2018). Mouse double minute homologue 2 (MDM2) downregulation by miR-661 impairs human endometrial epithelial cell adhesive capacity. Reprod. Fertil. Dev. 30 477–486. 10.1071/rd17095 [DOI] [PubMed] [Google Scholar]
- Wright K., de Silva K., Purdie A. C., Plain K. M. (2020). Comparison of methods for miRNA isolation and quantification from ovine plasma. Sci. Rep. 10:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Zhang Y. W., Tong X. H., Liu Y. S. (2015). Characterization of microRNA profile in human cumulus granulosa cells: identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol. Cell. Endocrinol. 404 26–36. 10.1016/j.mce.2015.01.030 [DOI] [PubMed] [Google Scholar]
- Xu H., Wang X., Wang Z., Li J., Xu Z., Miao M., et al. (2020). MicroRNA expression profile analysis in sperm reveals hsa-mir-191 as an auspicious omen of in vitro fertilization. BMC Genomics 21:165. 10.1186/s12864-020-6570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Gu W. W., Gu Y., Yan N. N., Mao Y. Y., Zhen X. X., et al. (2018). Association of the peripheral blood levels of circulating microRNAs with both recurrent miscarriage and the outcomes of embryo transfer in an in vitro fertilization process. J. Transl. Med. 16:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Schuster A., Tang C., Yu T., Ortogero N., Bao J., et al. (2016). Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development 143 635–647. 10.1242/dev.131755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang H., Jiang Y., Xue B., Diao Z., Ding L., et al. (2015). MicroRNA-181a is involved in the regulation of human endometrial stromal cell decidualization by inhibiting Krüppel-like factor 12. Reprod. Biol. Endocrinol. 13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zacur H., Cheadle C., Ning N., Fan J., Vlahos N. F. (2012). Effect of luteal-phase support on endometrial microRNA expression following controlled ovarian stimulation. Reprod. Biol. Endocrinol. 10:72. 10.1186/1477-7827-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Zhang D., Yang Y. U., Cui X., Sun J., Liang C., et al. (2017). MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and α 1, 3-fucosylation. Cell Death. Differ. 24 2161–2172. 10.1038/cdd.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., De Iuliis G. N., Dun M. D., Nixon B. (2018). Characteristics of the epididymal luminal environment responsible for sperm maturation and storage. Front. Endocrinol. 9:59. 10.3389/fendo.2018.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Stanger S. J., Anderson A. L., Bernstein I. R., De Iuliis G. N., McCluskey A., et al. (2019). Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 17:35. 10.1186/s12915-019-0653-5 [DOI] [PMC free article] [PubMed] [Google Scholar]