Abstract
Somatostatin receptor-targeted alpha radionuclide therapies have been introduced including actinium-225 labeled tetraazacyclododecanetetraacetic acid–octreotide (Ac-225 DOTATATE) for advanced metastatic neuroendocrine tumors (NET). Very limited data are available on the posttherapy imaging using Ac-225 DOTATATE therapy in metastatic NET. We present Ac-225 single-photon emission computed tomography/computed tomography images of a 72-year-old patient diagnosed as a case of advanced rectal NET treated primarily with Ac-225 DOTATATE.
Keywords: Actinium-225 tetraazacyclododecanetetraacetic acid–octreotide, liver metastases, rectal neuroendocrine tumor, single-photon emission computed tomography/computed tomography
Introduction
Neuroendocrine tumors (NET) are heterogeneous group of tumors with different malignant potentials.[1] Peptide receptor radionuclide therapy (PRRT) using somatostatin analogs labeled with beta (β)-particle emitting isotopes such as yttrium-90 and lutetium-177 (Lu-177) has been a promising treatment strategy for metastasized NET.[2] Although remission can be accomplished in a high percentage of NET, some tumors do not respond to this treatment. Alpha (α)-emitting isotopes such as actinium-225 (Ac-225) are characterized by extremely high cytotoxic activity on the cellular level and may be superior in the treatment of NET not responding to PRRT using β-emitting isotopes.[3] Furthermore, imaging α-emitting nuclides with such low count statistics and gamma rays using single-photon emission computed tomography/computed tomography (SPECT/CT) is not reported yet. Our case describes the image findings of Ac-225 tetraazacyclododecanetetraacetic acid–octreotide (DOTATATE) whole body and SPECT/CT in a case of rectal NET with metastases.
Case Report
A 72-year-old male presented with complaints of epigastric burning sensation, loss of appetite, constipation, and altered bowel habits. Ultrasound abdomen showed a pararectal mass and liver lesions. Upper gastrointestinal (GI) endoscopy showed Grade II esophagitis. Colonoscopy showed normal colonic study with extraneous impression in the rectum. CT contrast abdomen showed a cystic and solid complex mass lesion in the right lateral pelvic wall and precoccygeal region measuring 8.2 cm × 7.7 cm causing extrinsic compression of the rectum, retroperitoneal lymph nodes, and multiple liver metastases. Biopsy of pararectal lesion was consistent with NET Grade II (Ki-67-proliferation index-4%). His complete blood counts, liver function test, and kidney function tests were within normal limits. He was referred for whole-body gallium-68 (Ga-68) DOTANOC positron-emission tomography/CT (PET/CT) [Figure 1a, maximum intensity projection] for staging which showed intense uptake in enhancing lesion in right pararectal lesion [Figure 1b], retroperitoneal lymph nodes [Figure 1c], liver lesions [Figure 1d], and right third rib [Figure 1e] metastases. He was treated with 5.5 MBq (150 μCi) of Ac-225 DOTATATE as the primary modality of treatment with aminoven infusion. Posttherapy images were acquired at 24 h. High-energy general-purpose collimators were used for both whole body and SPECT/CT. Both scans were acquired using 218- keV and 440-keV photon energies with 20% window width. Whole-body images were acquired for 30 min using 256 × 1024 matrix [Figure 2]. SPECT/CT scan was acquired with sixty projections for 60 s using 64 × 64 matrix. Whole body [Figure 2] shows increased Ac-225 uptake in the pararectal lesion, lymph nodes, and liver metastases. SPECT/CT images of the pelvis [Figure 3a-d] show increased accumulation of Ac-225 DOTATATE in the pararectal mass lesion, concordant with Ga-68 DOTANOC PET/CT. The patient is on follow-up without any side effects.
Figure 1.
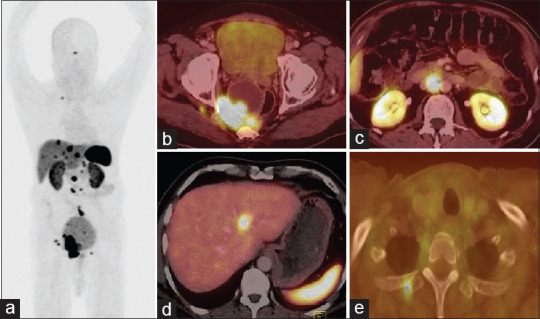
Gallium-68 tetraazacyclododecanetetraacetic acid–octreotide positron-emission tomography/computed tomography maximum intensity projection image (a) and axial fused positron-emission tomography/computed tomography images, showed intense somatostatin receptor expression in the pararectal lesion (b), aortocaval nodes (c), liver lesion, (d) and rib metastases (e)
Figure 2.
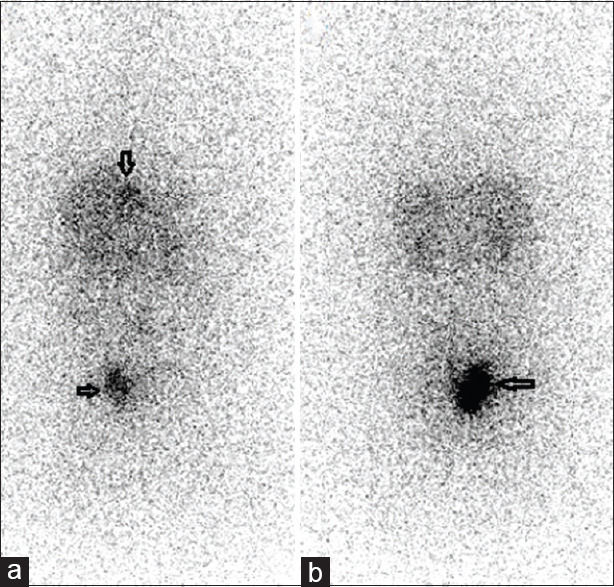
Whole-body posttherapy actinium-225 tetraazacy clododecanetetraacetic acid–octreotide anterior (a) and posterior (b) images showing intense accumulation in pararectal lesion and liver metastases (arrows) (note there is no bladder activity)
Figure 3.
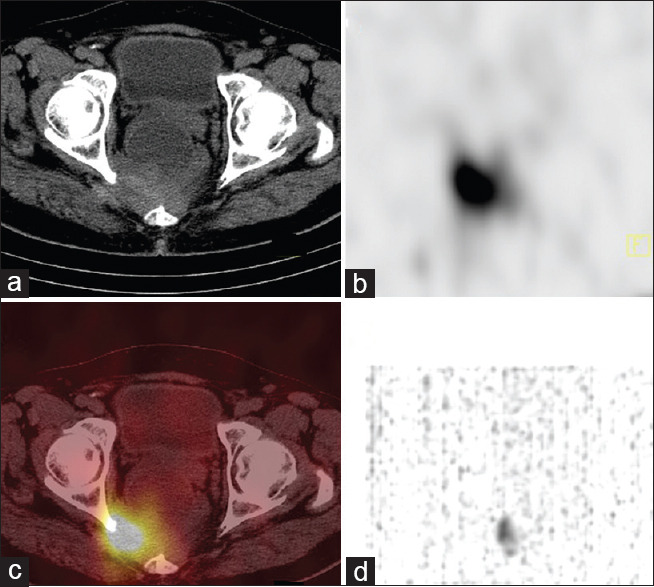
Actinium225 tetraazacyclododecanetetraacetic acid–octreotide singlephoton emission computed tomography/computed tomography (axial computed tomography (a), singlephoton emission computed tomography (b), fused singlephoton emission computed tomography/computed tomography (c) and Maximum intensity projection(MIP) image(d) showing intense uptake in the right pararectal lesion
Discussion
NETs have proven to be ideal neoplasms for PRRT, as the majority of these slow-growing malignancies overexpress somatostatin receptors. Appropriate candidates for PRRT are patients presenting with well-differentiated or moderately differentiated NET defined as NETs of Grade I or II according to the 2010 WHO classification.[4] The clinical presentation may vary depending on the site of tumor origin. About 72% of NETs arise in GI structures, 25% are bronchopulmonary in origin, and <5% arise at other sites (e.g., thymus, breast, and genitourinary system). Frequently, these tumors are discovered when metastatic or locally advanced and therefore inoperable.[5]
Functional imaging procedures using PET/CT with Ga-68 labeled somatostatin analogs are used for staging, assessing somatostatin receptor status, and making decisions on the most appropriate therapy regimens.[6] PRRT with Lu-177-DOTATATE is the first radiopharmaceutical approved by the Food and Drug Administration for NETs. The approval was based on the results of phase III, randomized controlled landmark international, multicenter open-label NET therapy (NETTER-I) trial, which compared Lu-177 DOTATATE versus high-dose long-acting octreotide in patients with inoperable progressive, G1–G2 somatostatin receptor-positive midgut NETs. The median progression-free survival had not yet been reached in the Lu-177 DOTATATE group at the time of interim analysis and was 8.4 months in the control group. The data support the efficacy of Lu-177 DOTATATE in Gastroenteropancreatic GEP-NETs.[7] The limitation of Lu-177 DOTATATE therapy is that 26%–55% of patients only achieve stabilization of disease and a significant number, i.e., 18%–32% are refractory to β radiation Lu-177 DOTATATE therapy. Moreover, those who achieve stabilization of disease invariably relapse in 2–3 years of the initiation of treatment.[8]
Ac-225 (half-life 9.9 days) for targeted α-therapy has distinct advantages including the short range of α radiation in human tissue, which allows the selective killing of targeted cancer cells while sparing surrounding healthy tissue, and much higher linear energy transfer than β-emitters, which leads to highly effective cell killing through DNA double-strand and DNA cluster breaks. The theoretical physical advantages of α-radiation over β-radiation improve the efficacy of PRRT by labeling the peptides with α-particle emitters.[9] The first study by Ballal et al. which is a prospective single-arm study in advanced NET patients who were refractory to Lu-177 DOTATATE demonstrated Ac-225 DOTATATE therapy to be effective in inducing high response rates, has a low toxicity profile and improves the quality of life.[10] We treated our patient with Ac-225 DOTATATE as the primary modality of treatment.
Limited data are available for Ac-225 posttherapy images. Posttherapy images are a potential source of quality control to confirm the localization of Ac-225 in the target metastatic sites and to calculate organ dosimetry wherever required. Current literature suggests clinical imaging using two photopeaks 440 KeV (26.5% of 213Bi decays to give 440 KeV) and 218 KeV during Ac-225 therapy [decay of Ac-225, Figure 4].[11] Recently, only one case of Ac-225 DOTATATE SPECT/CT image is published as image of the month by Ocak et al.[12] This is the first case report showing the whole body and SPECT/CT Ac-225 DOTATATE images.
Figure 4.
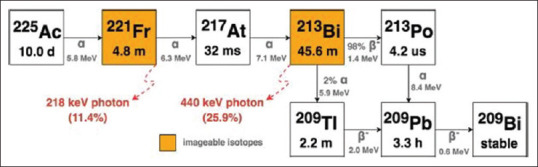
Actinium-225 decay showing 218 keV and 440 keV photon emission for imaging
Conclusion
Ac-225 DOTATATE can be used as a primary modality of treatment in advanced NET with metastases. Furthermore, it has the potential to use in Lu-177 DOTATATE resistant cases to promote remission without much toxicity. Posttherapy images are very important to see the biodistribution of tracer and for dosimetry purposes. As the dose of Ac-225 is used in microcurie range, imaging low counts needs more time for SPECT imaging. Using 218 keV and 440 keV gamma rays, whole body and SPECT/CT can be acquired with high energy collimator, posttherapy with Ac-225 DOTATATE.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–51. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46:723–34. doi: 10.1080/02841860701441848. [DOI] [PubMed] [Google Scholar]
- 3.Miederer M, Henriksen G, Alke A, Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R, et al. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res. 2008;14:3555–61. doi: 10.1158/1078-0432.CCR-07-4647. [DOI] [PubMed] [Google Scholar]
- 4.Rindi G. The ENETS guidelines: The new TNM classification system. Tumori. 2010;96:806–9. doi: 10.1177/030089161009600532. [DOI] [PubMed] [Google Scholar]
- 5.Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–16. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–10. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 7.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu- DOTATATE: The IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35. doi: 10.1007/s00259-011-1902-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy using 225Ac-DOTATOC achieves partial remission in a patient with progressive neuroendocrine liver metastases after repeated β-emitter peptide receptor radionuclide therapy. Clin Nucl Med. 2020;45:241–3. doi: 10.1097/RLU.0000000000002915. [DOI] [PubMed] [Google Scholar]
- 10.Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with 225 Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177 Lu-DOTATATE PRRT:First clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47:934–46. doi: 10.1007/s00259-019-04567-2. [DOI] [PubMed] [Google Scholar]
- 11.Rasheed R, Usmani S, Naqvi SA, Alkandari F, Marafi F. Alpha therapy with 225 actinium labeled prostate specific membrane antigen: Reporting new photopeak of 78 kilo-electron volts for better image statistics. Indian J Nucl Med. 2019;34:76–7. doi: 10.4103/ijnm.IJNM_115_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocak M, Toklu T, Demirci E, Selçuk N, Kabasakal L. Post-therapy imaging of 225Ac-DOTATATE treatment in a patient with recurrent neuroendocrine tumor. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-04725-x. doi: 10.1007/s00259-020-04725-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


